Abstract
Background: There is an increasing number of patient’s undergoing kidney transplantation in the United States. Kidney recipients have high occurrence of 30-day readmissions that leads to high hospital costs and decreased quality of life. Previous research found that a high level of post-transplant anxiety is correlated with increased likelihood of 30-day readmissions. The goal of this paper is to describe the study design and implementation process of a randomized control trial (RCT) using a standardized post-transplant mentoring program in order to reduce 30-day readmission and post-transplant anxiety among kidney transplant recipients.
Methods/Design: A single institution RCT evaluating post-kidney transplant patient’s anxiety level and readmission rates (both 30- and 90-day). The intervention group will consist of a standardized mentoring process during a four-week period following transplantation and the control group will undergo routine post-operative (PO) care. The mentors will be prior kidney-transplant recipients who will undergo a standardized training process. They will contact the intervention group at week intervals to help counsel on proper PO care and give routine advice. Objective and subjective data will be collected at week intervals for all participants over a one-year study period.
Discussion: Standardizing a post-transplant mentoring process has the possibility of improving transplant recipient quality of life, reducing post-transplant anxiety and result in fewer readmission rates.
Keywords
patient mentoring, standardized training, kidney transplantation, readmission rates, post-transplant anxiety
Abbreviations
RCT: randomized control trial; PO: post-operative; HCAHPS: Hospital Consumer Assessment of Healthcare Providers and Systems; OSUWMC: Ohio State University Wexner Medical Center; STAI: State-Trait Anxiety Inventory; MBOE: Master of Operational Excellence; POD= post-operative day; PD= post-discharge from initial transplant
Trial registration
Protocol Record 2017H0190. Registered 6 April 2018 - Retrospectively registered,https://clinicaltrials.gov/ct2/show/NCT03490188?term=2017H0190&rank=1
Trial registration: Protocol Record 2017H0190
Introduction
In 2014, over 17,000 patients received kidney transplants in the United States [1]. The wait list for such transplants is longer than 100,000 with more than 3,000 patients being added to the list every month [1]. Post-transplant, kidney recipients have high occurrence of 30-day readmissions. In a study covering kidney transplants from 2001 to 2005 they found the readmission rate to be 31% [2,3]. Proper self-care by patients after discharge, including adherence to medication protocols, doctor visits, dietary modifications, and infection prevention, plays an important role in preventing readmissions [4,5]. The instructions for such post-discharge (PD) care are given to the patients during their transplant-related stay at the hospital. However, these instructions for kidney transplant recipients are becoming more complex with the aggressive use of marginal organs, and with increasingly complicated transplant cases being accepted [6,7]. In addition, with early discharges stemming from added pressures for reducing patient lengths of stay [8], the scope of patient responsibilities for self-care is also increasing. This added complexity and content of post-transplant self-care leads to an increase in patient anxiety with respect to their conditions immediately following discharge.
An earlier study conducted by our group found that standardization of discharge work done by transplant nurses improved better quality of care, reduced anxiety, and reduced the occurrence of 30-day readmission rates [9]. Specifically, those analyses suggest that the likelihood of 30-day readmission was about one-third lower for kidney transplant recipients’ post-implementation of the standardized discharge process compared to recipients in a different yet comparable transplant process. This resulted in an estimated average annual savings of $133,346 for the hospital. There is also a 10% increase in the overall patient satisfaction for the treatment group, measured using the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, compared to the control group [10]. During a feedback session with a group of patients, it was noticed that patients were encouraged to hear from former transplant patients on how to navigate their first month of post-transplant. A follow-up conversation with additional patients reinforced this idea that transplant recipients can benefit through additional reinforcement from former transplant recipients on certain (non-medical) aspects of discharge instructions.
To minimize patent anxiety and occurrence of preventable readmissions in kidney transplant patients, we plan to demonstrate the effectiveness of standardization with regards to a patient mentoring program. Although the concept of patient mentoring is well established and practiced across several medical specialties, their effectiveness remains non-conclusive. We argue that a primary reason for this would be the lack of standardization to this intervention. By standardization, we refer to the interactions to be focused on specific content related to discharge compliance, timed and have a measurable outcome at the end of each interaction.
Objectives
In this research, we seek to examine the efficacy of using former patients as mentors to newly transplanted patients and its relationship with patient anxiety following discharge as well as the prevention of readmissions for kidney transplant recipients. Our primary objective is to evaluate preventable readmission rates among transplant patients. We hypothesize that the treatment group of patients who undergo a targeted mentorship program will be have lower preventable 30- and 90-day readmission rates. Our secondary objective is to evaluate patient anxiety scores. We hypothesize that patient anxiety among patients with mentoring will have lower anxiety scores than those without mentoring. Evidence from this experiment can help advance our understanding of patient-engagement in the continuity of care delivery; decrease patient readmission rates, and improves overall cost utilization.
Methods
We will conduct a single institution randomized control trial that assigns patients getting discharged after a kidney transplant to a treatment group that involves a 30-day mentoring initiative by former transplant patients and comparing the efficacy with a similar control group who undergoes only the standard discharge protocol. The overall study period will be divided into the following sections, summarized in Figure 1. Our protocol adheres to the SPIRIT guidelines.
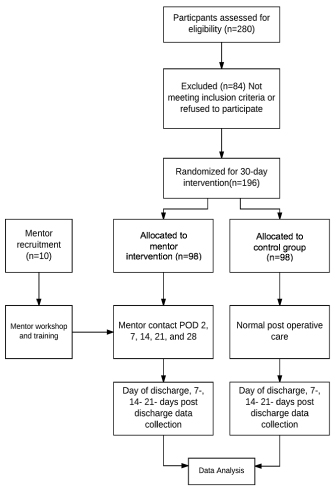
Figure 1. Consort flow diagram of trial timeline
Flow diagram depicting all four stages of the proposed trial including enrolment, intervention allocation, follow-up, and analysis
In order to address our research question the study design will involve a controlled experiment ranging over one year at the Ohio State University Wexner Medical Center (OSUWMC), a Comprehensive Transplant Center. The Transplant Center is a subdivision of the Department of Surgery at OSUWMC, which performs kidney transplants for patients from all over the United States. OSUWMC is an academic tertiary care center in Columbus, Ohio. Table 1 shows the basic demographics and the volume of the patients who had kidney transplants at the Transplant Center during the years 2014 and 2015. The institution specific 30-day readmission rate for kidney transplant patients averages approximately 25% (the national average is 33%) [11].
Table 1. Basic Demographics Kidney Transplant Recipients from 2014 and 2015
|
2014, n (%)
|
2015, n (%)
|
Age (years)
18 to <40
40 to <54
≥ 55
|
45 (22)
80 (40)
79 (38)
|
34 (22)
63 (40)
62 (37)
|
Gender
Male
Female
|
128 (62)
76 (38)
|
94 (60)
65 (40)
|
Race
White
African American
Asian
Other
Unknown/ not reported
|
146 (71)
53 (26)
2 (1)
3 (2)
1 (<1)
|
107 (74)
40 (22)
8 (1)
3(1)
1(<1)
|
Transplant Donor Kidney Type
Living Donor
Cadaveric
|
97 (47)
107 (5
|
64 (40)
95 (60)
|
Total
|
204
|
159
|
Recruitment
Mentor Recruitment
The transplant mentors will be recruited during a two-month period from the pool of former kidney transplant patients from the OSUWMC with the aim to recruit up to 10 mentors based on gender (including both male and female), race (Caucasian, African-American, and Asian), and age (broken into two age groups <45 years and >45 years) in order to reflect that of recipients in the transplant center. The mentors will be selected based on input from the coordinators, nephrologists, and social workers that worked with the recipients after their transplantation and still follow-up with care at OSUWMC. Inclusion criteria for mentors will include; transplant in the last 18-36 months and in good health condition after discharge.
Standardization of Patient Mentoring Workshop
A standardized patient-mentoring program was developed and planned by the research team. The training will occur through a half-day workshop. The workshop will be constructed with the first 15 minutes dedicated to discussing the need for discharge instruction reinforcement. This will then be followed by an hour-long session on measuring patient-mentor phone conversations and standardization of discharge topics. The discharge topics will include; timing of medications, compliance to medication regimens, compliance to doctor’s appointments, reassurance for calling doctors and transplant coordinators if there is an issue, frequency of lab draws, and water and fluid intake instructions. There will be a 45-minute summary and follow-up plan session followed by a 90-minute session measuring the patient-mentor phone conversation. This session is intended to help standardize the phone conversation mentoring discussion and give additional training for the mentors to help manage calls from the mentees. A final review and warp-up session to finalize all topics covered during the mentorship workshop concluded the session.
Participant Recruitment
The criterion for choosing the participants in the study will be based on their involvement in the discharge process following the kidney transplant at OSUWMC. Overall participant duration will be one year and is summarized in Table 2. Inclusion criteria was set to include any patient who received a kidney transplantation, deceased or living donor, during the study period. Exclusion criteria include anyone younger than 18 years old or older than 70 years old. The lower limit age restriction intends to exclude children given their transplant process varies from adults. The upper limit age restriction is in place due to the small number of patients receiving a transplant after 70 years of age. We expect about 280 patients will be eligible for during the year long period of the study implementation. Of those, an estimated 70% of patients will consent for study participation. Based on the data from last two years of transplant at OSUWMC, we expect that the mean age of eligible patients will be about 50 years and about 42% will be female. In addition, about 70% will be white non-Hispanic, 25% black non-Hispanic, 1% Asian, and 1% another race/ethnicity.
Table 2. Individual Participant timeline during study protocol period
SPIRIT flow diagram for the predicted timeline for each individual participant starting with recruitment and ending at 30 days post-discharge from transplant
|
STUDY PERIOD (30-days per participant)
|
|
Enrolment
|
Allocation
|
Post-allocation
|
Close-out
|
TIMEPOINT**
|
Admission for Transplant
|
0
|
POD* 2
|
7-days PD**
|
14-days PD**
|
21-days PD**
|
28-days PD**
|
30 days PD
|
ENROLMENT:
|
|
|
|
|
|
|
|
|
Eligibility screen
|
X
|
|
|
|
|
|
|
|
Informed consent
|
X
|
|
|
|
|
|
|
|
Transplant
|
X
|
|
|
|
|
|
|
|
Allocation
|
|
X
|
|
|
|
|
|
|
INTERVENTIONS:
|
|
|
|
|
|
|
|
|
Mentor Contact
|
|
|
X
|
X
|
X
|
X
|
X
|
|
ASSESSMENTS:
|
|
|
|
|
|
|
|
|
30-Day Readmission Rate
|
|
|
|
|
|
|
|
X
|
STAI Stress Survey
|
|
|
X
|
X
|
X
|
X
|
X
|
|
Objective Measures Participants***
|
|
|
X
|
X
|
X
|
X
|
X
|
|
Objective Measures Participant and Mentor***
|
|
|
X
|
X
|
X
|
X
|
X
|
|
*POD= post-operative day, **PD= post-discharge from initial transplant, *** Objective Measures listed in main text
To recruit participants for this study, both patients and their families will be given all the information regarding the study at the time of admission for transplant by the inpatient nurse team. At this time, the project will be described to the patient and their family, eligibility will be confirmed, and the transplant recipient will be invited to participate.
Informed Consent
If the patient is interested in participating, they will be given more details about the project’s goals, tasks, time required, and potential risks. If a patient prefers, this discussion can be held in a private room upon admission. The following points will be emphasized: (1) project participation is voluntary, (2) failure to participate will not adversely affect the patient’s medical care, (3) the patient can withdraw from the study at any point, (4) no identifying data about the patient will be publicly released, (5) it is not known if participation in the study will lead to improved benefits upon discharge, and (6) the information obtained may be shared with the patient’s nephrologists and other healthcare providers. The research transplant nursing team will then obtain written consent for participation from interested patients. An example informed consent is attached in the appendices. The patient will be provided with the institution’s approved IRB application during the time of enrollment.
Allocation
There will be a 1:1 allocation between intervention group and control group. As patients are successfully recruited, patients allocated to the treatment group will be assigned to a mentor matched on ethnicity and age group. Allocation will be performed by a computer algorithm using dynamic allocation. The research team will be using a health literacy tool to facilitate the matching process.
Intervention
The control group will only undergo the standard post-transplantation discharge care that includes formal discharge instructions from a trained discharge transplant coordinator, weekly follow-up visits and 24-hour access to transplant triage call center. If the patient is assigned to the treatment group, they will receive the normal post-transplantation discharge care and be assigned a mentor. The first contact will be made by the mentor with their assigned patient on post-operative (PO) transplant day 2 via video chat while the transplant recipient is still inpatient. The transplant discharge coordinator will be present to introduce the mentor to the patient and facilitate introductions. The primary purpose of the first call is to build relationship with the patient. The second contact will be made after the discharge when the transplant coordinator calls the patient (typically 48 hours after discharge). After this point, there will be four standardized mentoring touch points (one every week) that will be approximately 30 minutes long. Each week a different topic will be discussed during the phone calls;
Week 1: Medications
Week 2: Lab work
Week 3: Water and fluid intake/balance
Week 4: Adherence to doctor’s appointments
These themes correlate with the training sessions for the mentor’s during their training workshop.
Follow-up
Transplant recipients are required to return to the post-transplant center for follow-up every week after discharge for the first month. Both objective and perceptual data from the study participants will be collected at each weekly follow-up visit for the first month. Thus, patient enrollment in the study will occur over a 1-month timeframe while recruitment will occur over 12 months. This will allow for approximately 200 transplant patients for possible enrollment and query.
Data collected and reported outcomes
Patient-perceived outcomes will be evaluated using the State-Trait Anxiety Inventory (STAI) for Adults. It will be administered to measure baseline and current anxiety and stress levels over the course of the treatment period. The survey will be administered 2 times; at time of discharge prior to leaving the hospital and at 31 days during scheduled clinic visits. Objective data will also be collected including; readmission rates, discharge-readmission time, length of stay upon readmission, reason for readmission, and graft rejection rates. In the case of a readmission to the hospital, a different survey will be used to collect some additional objective data regarding patient behavior in an attempt to identify the cause of readmission. The team will focus on compiling the findings and preparing interim project summaries.
Both objective and perceptual data will be collected at different stages of the study. Data will be collected from different participants in the study including patients, transplant nurses, and administrators. Details on the information to be collected from different recipients are included below. The actual scale/survey instruments are provided in patient survey. The survey items are thoroughly vetted for reliability and validity using published methods, and using data collected from a convenience sample of students from the Master of Operational Excellence (MBOE) program before the first standard work session.
Data Collected from Patients
The following data will be collected from patients and family members of patients who are getting discharged after a kidney transplant:
Dependent Variables
Perceptual measures will be collected via the STAI for perceived stress at day of discharge, 14 days and 31 days post-transplant in person during lab visits.
Multiple objective performance measures will be collected from each of the participating hospitals. Readmission rates with the primary cause for readmission at 30- and 90-day for kidney transplants will be collected. A secondary survey will be administered at the time of readmission for all the patients (treatment and control) enrolled in the study in order to identify the factors that contributed to readmission (e.g. missed labs or medications, financial burden, patient-mentoring, etc.)
The duration of stay for a kidney transplant patient upon readmission will also be collected. The median length of stay reflects a number of different performance dimensions, including cost [12], quality of care [13], profitability [14], and healthcare efficiency [15]. Other measures to be collected include; the 30 and 90-day percentage of cases ending up with a loss of function of the transplanted kidney, the timing of graft rejections/failures, the 30 and 90-day PD mortality rates, the causes of death, the LACE readmission risk score at time of discharge, the number of times patients missed clinic visits, the number of times patients missed lab draws and how many times patients missed two consecutive labs, the number of times the patients visited the Emergency Room through the period of the study. The incidence of ongoing and new-onset diabetes, wound-site or systemic infections, and serious adverse cardiac events through the first month of patient follow-up will also be assessed [16].
Independent Variables
The status of whether the patient is in the control or treatment group.
Control Variables
The following patient characteristics that will be controlled during the study include; race (includes ethnicity), gender, age group, annual household income level, highest level of education, donor type (living or cadaver), and diabetes status at time of transplant.
Data collected from the Patients and Mentors
To assess the reliability of mentoring interactions, a recording tool will be used to collect information from both the patient and mentors. This will include date when contact takes place, length of time of each contact, mode of contact (in person, phone, email, video-chat, etc.), topics discussed (including both what is advocated for each session and other discussion points not enumerated in the provided list).
Outcome
Primary outcome is 30- and 90-day preventable hospital readmission rate. Preventable readmissions refer to occurrence of readmissions due to the following reasons: missing two consecutive labs, clinic visits, and lack of medication compliance. This information will be collected from the enrolled patients (across both arms) who are readmitted within 30-days after discharge. The secondary outcome is patient anxiety scores at three-time periods (time of discharge, 14 days and 31 days after discharge).
Internal Validity Measures/Avoidance of Bias
Multiple methods will be in place so as to minimize bias in the study design and process. First, all patients will be randomly assigned to the treatment or control group so as to avoid confounding bias. Second, for patients who are not willing to participate in our study, we will collect basic demographic data to compare with the responding group to test for any respondent bias attributable to demographic factors. Third, both the treatment and control groups will have the same number of members in order to avoid team size effects.
Fourth, sample size is based off of the previous 2 years of data and an estimate of the number of kidney transplant patients who will undergo discharge using Treatment or Control Group standardized work during the study period. A statistical power analyses (detailed below) was performed and projected numbers for participants confirm a high likelihood that sufficient patient participants will be recruited. This will allow accurate sample size to discern any meaningful differences in patient outcomes between the treatment and control patient groups.
Sample Size
Assuming 30 patients per arm, we would be powered at 90% to detect a 1 standard deviation difference between continuous outcomes on patient anxiety. All calculations are based on a two-sided test with an alpha of 0.05. To ensure there was adequate support for all our mentors, a maximum of 35 patients can be randomized into the treatment arm.
Statistical Methods
All patient outcomes will be analyzed in the arm to which they were assigned during randomization. Baseline characteristics of the patients in terms of length of stay, pre-existing conditions, age, gender will be summarized with each trial arms to provide counts and frequencies for categorical variables and means with standard deviations and ranges for continuous variables. We will first examine the null hypotheses of no difference between arms in baseline characteristics using t-tests and chi-square tests adjusted for clustering by the mentors.
To account for the effects of mentors, we will use hierarchical generalized linear models with random main effects specified at the mentor level. Such models are appropriate for analyzing cluster randomized trials and will allow us to account for any imbalances in characteristics across study arms. For measures collected from the patients, the outcome of interest will be the 30-day post discharge -baseline responses, adjusting for baseline responses. If there are differences in baseline characteristics between the two study groups, these will be accounted for using hierarchical generalized logistic or linear regression models that include an indicator for study arm.
If the Treatment Group patient outcomes prove significantly better than the Control Group outcomes, this knowledge will be transferred to other surgical procedures (e.g. hip and knee replacements) on guidelines and tools for designing patient-centric processes. Control Group patient objective outcomes will also be compared to retrospectively reviewed historical outcomes from 2014-2015, to determine whether the patient-mentor program has an impact on post-transplant outcomes compared to current OSUWMC discharge protocols.
Detailed study procedures and Safety
Several methods will be in place to ensure the safety and monitoring of the intervention. First, the intervention is theoretically sound and based on prior work at the institution and that of others. Second, the survey instruments will be refined and pre-tested prior to the beginning of the study. This will help assess the reliability and validity of the instruments. Third, the principal investigator will meet at least weekly with other study personnel in order to trouble-shoot any potential areas of concern regarding the safety of the study protocol. Fourth, all transplant nurses will undergo centralized training. Fifth, transplant nurses will be closely monitored through a combination of site visits, phone calls, and electronic mail. Sixth, project data will be reviewed quarterly by the study team. Any indications of potential threats to patient safety or a pattern of refusal by patients to participate in specific interactions with nurses will be responded to immediately by a careful review conducted by the study team. When appropriate, involvement of the patient’s nephrologist or dialysis facility medical director, reporting to our Institutional Review Board, and adjustment of the project protocol will occur. Although risk to subjects is considered minimal, all unanticipated adverse events will be reported to our Institutional Review Board.
To protect patient privacy confidential patient data, demographic characteristics and survey responses are stored on separate password protected files to minimize participant privacy concerns. Further all patient level identifiers will be removed before data is made available for analysis. To protect against breach of confidentiality all project data will be kept on a password protected server at OSUWMC that is automatically backed up. No identifying data about participants will be publicly released or reported in articles resulting from this study. Data will only be accessed by the PI, CO-PI and Key Personnel. The oversight will be provided by the OSU HIPPA regulations as part of our bylaws and federal statutes.
As soon as required contribution from the patient towards the study is complete, identifiers specific to that patient will be removed and de-identified information will be used for subsequent analysis. Once all contribution of the patient to the research is complete survey responses from the patient will be linked to demographic and pertinent medical history. Once the data has been linked the patient will be assigned a generic id and any identifiers like the patient medical records will be removed from the database.
Discussion
Standardized self-mentoring among kidney transplant recipients is an innovative method to help reduce the number of readmission rates among transplant patients. It also has the potential in minimizing post-surgical anxiety for other procedures. Furthermore, these reduction in readmission rates have the potential to decrease overall hospital costs, improve patient satisfaction and improve short term post-transplant complication rates. This intervention will also allow for prior kidney transplant patient’s to be actively integrated into PO treatment plans and become advocates for new transplant recipients. It will also give transplant recipients a new, personalized resource to help with managing the overwhelming amount of information and minimize patient anxiety. Finally, this approach to use former transplant patients as mentors for non-medical related issues will also minimize the burnouts and turnovers among the outpatient nursing coordinators who constantly grapple with questions on compliance and reassurance from the transplant recipients [17]. This study will allow for measurement of both patient anxiety, readmission rates and standardization of the mentoring process with the potential to greatly improve quality of life in patient’s undergoing kidney transplantation.
There have been few similar studies that have evaluated post-hospitalization mentoring and readmission rates. However, these studies were performed in spinal cord injury patients [17] and chronic obstructive pulmonary disease patients [18], and did not specifically evaluate PO patients. Both studies saw improvement in patient self-efficacy and readmission rates with more intensive PD support systems. Our study will be the first large RCT evaluating a post-transplant standardized mentoring process to improve patient readmission and anxiety rates. Given the success seen in other patient populations, this mentoring program has the possibility of overall success in changing the PO period in our patient population.
There are several limitations to the study design. There is a concern for selection bias given both the mentors and the kidney recipients volunteer to be a participant in the study. However, there is a very rigorous vetting process for any patient that undergoes kidney transplantation and these patients are already a very specific subset. Furthermore, mentors are selected only if they are in good health standing. However, the goal of the mentoring process is to help with routine PO care and anxiety support, not to provide advice on PO complications. There is also concern about the standardization of the mentoring process. However, both central nursing and the mentors undergo a stringent training process and are censored during the intervention period. This will allow for continuous monitoring and screening for standardization among the intervention group. There is also the possibility for confounding bias; however, both the treatment and control group will undergo randomization prior to intervention. Furthermore, mentors and mentees will undergo matching so as to further limit any confounding bias. There is also the possibility of loss to follow-up given the prospective nature of this study; however, this is unlikely given the expectations that are set prior to transplantation and low loss to follow-up seen in routine patients. Furthermore, internal validity and reliability of the survey measures used in this study are a possible source of error. However, both the STAI [19] and the LACE score [20] have been previously validated in the literature and vetted by MBOE students prior to use in this particular study.
In conclusion, this is the first randomized control trial to look at PO kidney transplant peer mentoring and readmission rates. This study has the potential to improve early post-transplant quality of life and help decrease overall cost burden to both the patient and hospital system. Depending on final study data, this type of mentoring process can be possibly implemented at other transplantation centers nationwide in order to help decrease the burden of readmissions in this growing patient population.
Declarations
Ethics approval and consent to participate
This study was approved by The Ohio State University Institutional Review Board and Ethics committee (Approval #2017H0190).
Consent for publication
The Authors consent for publication of this material.
Competing interests
The authors declare that they have no competing interests.
Funding
This work has no formal funding contributions.
Authors' contributions
CH composed the body of the manuscript and created images. AC and SMB both contributed to editing and formatting of the manuscript and images. SH, YC, KH, MLH and PH helped in operationalizing the plan and manuscript edits.
Acknowledgements
The authors would like to acknowledge Kelly Toops, Janel Mullet and Molly Urbancic for their help with developing the protocol and their design effort.
Appendix
References
- USRDS (2012) 2018 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States; US Renal Data System, National Institutes of Health.
- Collins DBH (2018) Renal Transplantation. Medscape Reference: Drugs, Procedures, U.h.e.m.c.a.-o.
- Kiley DJ, Lam CS, Pollak R (1993) A study of treatment compliance following kidney transplantation, Transplantation 55: 51-56.
- Baker RJ, Mark PB, Patel RK, Stevens KK, Palmer N (2017) Renal association clinical practice guideline in post-operative care in the kidney transplant recipient. BMC Nephrol 18: 174.
- Ponticelli C, Cucchiari D, Graziani G (2011) Hypertension in kidney transplant recipients. Transpl Int 24: 523-533.
- Laederach-Hofmann K, Bunzel B (2000) Noncompliance in organ transplant recipients: a literature review. Gen Hosp Psychiatry 22: 412-424.
- Segev DL (2012) Innovative strategies in living donor kidney transplantation. Nat Rev Nephrol 8: 332-338.
- Ahttp://pcmh.ahrq.gov/portal/server.pt/community/pcmh__home/1483/PCMH_Home_Resources_for_Researchers_v2., AHRQ Publication No. 11-0023-EF
- Chandrasekaran A, Anand G, Sharma L, Pesavanto T, Hauenstein ML, et al. (2016) Moffatt-Bruce, Role of in-hospital care quality in reducing anxiety and readmissions of kidney transplant recipients. J Surg Res 205: 252-259.e1.
- Chandrasekaran A, Anand G, Ward P, Sharma SL, Moffatt-Bruce (2017) Design and Implementation of Standard Work on, C.D.P.A.Q.-E. Investigation.
- McAdams-DeMarco MA, King EA, Luo X, Haugen C, DiBrito S, et al. (2017) Frailty, Length of Stay, and Mortality in Kidney Transplant Recipients: A National Registry and Prospective Cohort Study. Ann Surg 266: 1084-1090.
- Polverejan E, Gardiner JC, Bradley CJ, Holmes-Rovner M, Rovner D (2003) Estimating mean hospital cost as a function of length of stay and patient characteristics. Health Econ 12: 935-947.
- Thomas JW, Guire KE, Horvat GG (1997) Is patient length of stay related to quality of care? Hosp Health Serv Adm 42: 489-507. [Crossref]
- Sear (1992) A.O.c.a.c.p. of, i.-o.m.s.H.H.S. Admin 37: 403-415.
- Burns LR, Chilingerian JA, Wholey DR (1994) The effect of physician practice organization on efficient utilization of hospital resources. Health Serv Res 29: 583-603. [Crossref]
- Neumann JL, Mau LW., Virani S, Denzen EM, Boyle DA, Boyle, et al., (2017) Burnout, Moral Distress, Work-Life Balance and Career Satisfaction among Hematopoietic Cell Transplantation Professionals. Biology of Blood and Marrow Transplantation.
- Gassaway J, Jones ML, Sweatman WM, Hong M, Anziano P, et al. (2017) Effects of Peer Mentoring on Self-Efficacy and Hospital Readmission After Inpatient Rehabilitation of Individuals With Spinal Cord Injury: A Randomized Controlled Trial. Arch Phys Med Rehabil 98: 1526-1534.e2.
- Benzo R, Vickers K, Novotny PJ, Tucker S, Hoult J, et al. (2016) Health Coaching and Chronic Obstructive Pulmonary Disease Rehospitalization. A Randomized Study. Am J Respir Crit Care Med 194: 672-680.
- Julian LJ (2011) Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A), Arthritis Care Res (Hoboken) 63 Suppl 11: S467-S72.
- van Walraven C, McAlister FA, Bakal JA, Hawken S (2015) Donzé J External validation of the Hospital-patient One-year Mortality Risk (HOMR) model for predicting death within 1 year after hospital admission. CMAJ 187: 725-733.

