This study investigated the concentrations of Cd and Pb in 18 vegetables (12 fruit types and 6 leafy types) and their habitat topsoils collected from three farming sites in Peninsular Malaysia. The levels of Cd and Pb are all significantly (P< 0.05) higher in the leafy vegetables than those in the fruit vegetables. It is found that the Cd levels in the vegetables are highly correlated with the three geochemical and non-resistant fractions of the habitat topsoils. This indicated that Cd geochemical fractions in the habitat topsoils are considered readily and potentially bioavailable to the vegetables. However, the Pb levels in the vegetables are very weakly (low) correlated with the all geochemical fractions of the habitat topsoils. Practically, there is no clear correlations for other pairwises for Pb. The positive relationships indicated the potential of edible vegetables as good biomonitors of Cd pollution in the habitat topsoils. For the health risk assessment, al the target hazard quotient values for Cd and Pb in all the vegetables investigated in both adult and children are all below 1.00, except for the THQ for Cd in the Amaranthus viridis and A. tricolor. These two vegetables were found to have Cd THQ higher than 1.00, indicating the potential non-carcinogenic risk of Cd to the consumers for both adults and children. Hence, the routine monitoring and management of the vegetable farms is recommended and necessary.
vegetables, target hazard quotient; Cd and Pb, biomonitor
Consumption of vegetables containing heavy metals is one of the main ways in which these elements enter the human body. After transferred to the human body, these toxic metals can be deposited in bones and overlapping noble minerals. Later, these metals can cause an array of diseases [1].
Heavy metal accumulation in agricultural vegetables is a growing major concern globally [2]. The concerns of health risks of metals in the edible vegetables has been raised in many publications such as mostly consumed vegetables from northern Bangladesh [3], leafy vegetables from Pearl River Delta, South China [4], a semi-urbanized area from Haryana state, India [5], Spinacia oleracea in Tshwane, South Africa [6], agricultural crops and homegrown vegetables in northern France [7], Kubanni River, Nigeria [8], and Spelter, WV, USA [9]. The human health risks of metals in food crops irrigated with wastewater have been widely reported from China [10-12].
Relationships of metal levels between vegetables and their habitat soils has been investigated based on vegetable and soil system in Chongqing, Southwest of China. [13], Bangladesh [14], and greenhouse vegetable cultivation from Kunming City (China) [15].
The objectives of this study are to 1) assess the concentrations of Cd and Pb in vegetables at three farming areas in Peninsular Malaysia, 2) assess the human health risks of Cd and Pb in the fruit and leafy types of vegetables, and 3) to assess the potential of vegetables as good biomonitors of Cd and Pb by studying the relationships and transfer factor of both metals between the vegetables and geochemical fractions of the habitat topsoils.
Study area and sampling
Eighteen species of vegetables were collected from Kg Ara Kuda (Penang), Kuala Ketil (Kedah) and Kg Sitiawan Manjung (Perak) of Peninsular Malaysia (Figure 1). At the same time, habitat topsoils (0-10 cm) from each vegetable habitat were also collected. They were then stored in clean polythene bags. All sampling of vegetables and their habitat topsoils were conducted within six months from September 2016 till January 2017.
Figure 1. Sampling map of vegetables from three farming areas in the northern part of Peninsular Malaysia.
In this study, the 18 vegetables with 12 fruit types and 8 leafy types were investigated (Table 1). The morphology and classification of the vegetables from selected vegetables have been identified according to Chin and Yap [16] and Prohens and Nuez [17,18].
Table 1. Description for sampling site of 18 vegetables collected from Kg Ara Kuda (Ara), Kuala Ketil (Ketil) and Kg Sitiawan Manjung (Manjung) of Peninsular Malaysia
No. |
Sites |
Vegetables |
Edible parts |
Sampling date |
Site description/Source of irrigation |
1 |
Manjung |
Allium tuborosum |
Leaves |
26-Oct-16 |
Agriculture and residential area/Domestic waste water |
2 |
Ara |
Amaranthus tricolor |
Leaves |
29-Sep-16 |
Agriculture area surrounded by palm oil plantation/Tube well and stream |
3 |
Ara |
Amaranthus viridis |
Leaves |
29-Sep-16 |
Road side of main road Penanti to Tasek Gelugor/Tube well and stream |
4 |
Manjung |
Brassica rapa |
Leaves |
26-Oct-16 |
Road side and residential area/Domestic waste water |
5 |
Manjung |
Ipomoea reptans |
Leaves |
9-Nov-16 |
Road side, less then 1km from coastal region/Tube well and stream |
6 |
Manjung |
Lactuca sativa |
Leaves |
9-Nov-16 |
Agriculture and residential area/Domestic waste water |
7 |
Ara |
Abelmoschus esculentus |
Fruits |
12-Oct-16 |
Palm oil plantation, main road/Tube well and stream |
8 |
Ara |
Benincasa hispida |
Fruits |
20-Oct-16 |
Main road Penanti to Tasek Gelugor/Tube well and stream |
9 |
Ara |
Capsicum annum |
Fruits |
20-Oct-16 |
Road side and surrounded by palm oil plantation/Tube well and stream |
10 |
Ara |
Cucumis sativus |
Fruits |
12-Oct-16 |
Main road Penanti to Tasek Gelugor/Tube well and stream |
11 |
Ketil |
Cucurbita moschata |
Fruits |
11-Jan-17 |
Residential area/Nearest stream |
12 |
Ketil |
Lagenaria siceraria |
Fruits |
21-Dec-16 |
Residential area/Nearest stream |
13 |
Ketil |
Luffa acutangula |
Fruits |
21-Dec-16 |
Road side of main road Baling to Petani River/Nearest stream |
14 |
Ara |
Momordica charantia |
Fruits |
12-Oct-16 |
Agriculture area surrounded by palm oil plantation/Tube well and stream |
15 |
Ketil |
Momordica charantia L. |
Fruits |
8-Dec-16 |
Private farm about 6 acres near residential area/Tube well and stream |
16 |
Manjung |
Solanum melongena |
Fruits |
17-Nov-16 |
Fisherman village/Domestic waste water and stream |
17 |
Ketil |
Tricosanthes celebica |
Fruits |
8-Dec-16 |
Residential area/Nearest stream |
18 |
Ketil |
Vigna sinesis |
Fruits |
8-Dec-16 |
Road side, in between Baling to Petani River/Nearest stream |
Preparation of vegetables samples
The vegetable samples were sorted in accordance with to their type of species. All samples brought to the laboratory for analyses. The collected samples were washed with distilled water to remove soil particles. Then samples were cut into small pieces using a clean knife then were dried in an oven at or 60°C until a constant dry weight. After drying, the vegetable samples were grinded into a fine powder using a commercial blender and stored in polyethylene bags, before used for acid digestion.
Preparation and treatment of topsoil samples
The collected samples will be dried in an oven at 100°C for 72 hrs until a constant dry weight. Later, the dried soils were grinded into a fine powder using a mortar and pestle, and they were sieved under 63 µm mesh size sieve. For the geochemical fractionations, the topsoils were fractionated into three fractions namely, first fraction as ‘easily, freely, leachable and exchangeable’ (F1), second fraction as ‘acid-reducible’ (F2), and third fraction as ‘oxidisable-organic’ (F3). The summation of F1, F2 and F3 will form the non-resistant (NR) fraction. The geochemical fraction analysis on the topsoils were based on Badri and Aston [19].
Determination of Cd and Pb
All samples stored in acid-washed pill box were analyzed by using atomic absorption spectrophotometer (AAS) model Thermo Scientific iCE 3000 series for Cd and Pb at the Faculty of Science, Universiti Putra Malaysia. Standard solutions were prepared from 1000 ppm stock solution provided by Sigma-Aldrich for both metals and data obtained from the AAS were presented in mg/kg dry weight basis.
Quality assurance and quality control
All the glass wares used in this study were acid-washed to avoid external contamination. Two certified reference materials (CRM) were used to check for the analytical procedures and accuracy of the method used. The CRM for Cd and Pb included were NSC DC 73319 for soil and Lagarosiphon major N.60 for vegetable. Based on the soil CRM, the recoveries were between 98.5 and 100.6% while the vegetable CRM were between 107.3 and 119.2% (Table 2).
Table 2. Comparisons of metal concentrations (mg/kg dry weight) between certified and measured values. The certified values were based on certified reference materials for soils (NSC DC 73319) and vegetables (Lagarosiphon major N. 60)
|
NSC DC 73319 (Soil) |
Lagarosiphon major N.60 (vegetable) |
|
Certified value |
Measured value |
Recovery (%) |
Certified value |
Measured value |
Recovery (%) |
Cd |
4.30±0.40 |
4.24±0.03 |
98.5 |
2.20±0.10 |
2.36±0.20 |
107 |
Pb |
98.0±6.00 |
98.6±2.00 |
101 |
64.0±4.00 |
76.3±2.40 |
119 |
Determination of water content, conversion factor and estimated daily consumption rate
Water content in the samples was calculated to determine the amount of moisture trapped in the samples until get the constant weight. The percentage of water content (WC) was calculated as below:
WC=(wet weight (g)- dry weight (g))×100%/wet weight (g)
The means values of conversion factor (CF) of the edible parts of 18 vegetables are presented in Table 3.
Data treatment
For the human health risk assessment, the present concentrations in dry weight (dw) basis were converted into wet weight basis because consumption (or cooking) of the vegetables are assumed to be in wet weight (ww). Therefore, the present concentrations (mg/kg dry weight) of Cd and Pb were converted to wet weight basis by using respective conversion factor for each vegetable, as shown in Table 3.
Table 3. Conversion factor (CF) of the edible parts of 18 vegetables from three farming sites in Peninsular Malaysia
No |
Vegetables |
CF |
1. |
Abelmoschus esculentus |
0.095 |
2. |
Allium tuborosum |
0.084 |
3. |
Amaranthus tricolor |
0.101 |
4. |
Amaranthus viridis |
0.080 |
5. |
Benincasa hispida |
0.052 |
6. |
Brassica rapa |
0.099 |
7. |
Capsicum annum |
0.091 |
8. |
Cucumis sativus |
0.043 |
9. |
Cucurbita moschata |
0.160 |
10. |
Ipomoea reptans |
0.100 |
11. |
Lactuca sativa |
0.068 |
12. |
Lagenaria siceria |
0.056 |
13. |
Luffa acutangular |
0.054 |
14. |
Momordica charantia |
0.061 |
15. |
Momordica charantia L |
0.046 |
16. |
Solanum melongena |
0.080 |
17. |
Tricosanthes celebica |
0.052 |
18. |
Vigna sinensis |
0.094 |
The estimated daily intake (EDI) value was calculated using the following formulas:
EDI=(Mc×CR)/BW
where;
Mc=the metal concentration in vegetables (mg/kg wet weight).
CR=the consumption rate of vegetables (345 g/day for adults and 232 g/day for children) and average body weight (55.90 kg for adults and 32.70 kg for children) respectively [20].
In this study, a non-cancer risk assessment method is based on the use of target hazard quotient (THQ), a ratio between the estimated dose of contaminant and the reference dose below which there will not be any appreciable risk. The THQ determined with the formula described by USEPA [21]:
THQ=EDI/ RfD
where;
EDI=estimated daily intake calculated previosuly;
RfD=the oral reference dose. The RfD (μg/kg wet weight/day) value for Cd used in this study was 1.00, provided by the EPA’s Integrated Risk Information System online database [22]. Since RfD for Pb was not available according to the EPA’s IRIS [22], the present study employed the RfD as 4.00 μg/kg wet weight/day as proposed by FAO/WHO [23]. It is estimated that if the THQ ratio is less than one (THQ < 1), the contaminated food or vegetable consumption do not result in high risk of adverse effect to human health.
Transfer factor
The transfer factor (TF) can be used to evaluate the potential capability of crops to transfer metals from soil to edible parts. It is defined as the ratio of the metal concentration in the edible part of crop to metal concentration in the habitat soil [14,24]. This factor represents the potential capability of heavy metals’ transmission from soil to the edible parts of vegetable [1,25]. The TF was calculated based on dry weight, as follows:
F=Cvegetable/Csoil
where;
Cvegetable=the metal concentration (mg/kg dry weight) in the vegetable;
Csoil=the metal concentration (mg/kg dry weight) in the geochemical fractions namely F1, F2, F3 and NR in the habitat topsoils.
Concentrations of Cd and Pb
The Cd concentrations (mg/kg dw) in the fruit vegetables range from 0.17 to 1.32 (mean: 0.69) (Table 4) while 0.74 to 2.17 (mean: 1.29) for the leafy vegetables (Table 5). The Pb concentrations (mg/kg dw) in the fruit vegetables range from 0.62 to 1.85 (mean: 1.19) (Table 6) while 1.23 to 2.74 (mean: 1.80) for the leafy vegetables (Table 7). The levels of Cd and Pb are all significantly (P< 0.05) higher in the leafy vegetables than those in the fruit vegetables. Based on the cited data from Li, et al. [24], the Cd and Pb concentrations (mg/kg ww) in the fruit vegetables range from 0.005 to 0.039 (mean: 0.018), and 0.015 to 0.075 (mean: 0.032), respectively (Table 8). For the leafy vegetables, the levels of Cd and Pb range from 0.035 to 0.078 (mean: 0.050), and 0.021 to 0.132 (mean: 0.080), respectively (Table 9). Therefore, Li, et al. (2012)’s findings supported the present results on the higher levels of Cd and Pb in the leafy than those in the fruit vegetables.
Table 4. The concentration (mean±SD, mg/kg dry weight) of Cd in the fruit vegetables, geochemical fractions of the habitat topsoils, and their transfer factors (Cd/F1, Cd/F3, Cd/F3, and Cd/NR) collected from three farming sites in Peninsular Malaysia
Fruit vegetables |
Cd |
CdF1 |
CdF2 |
CdF3 |
CdNR |
Cd/F1 |
Cd/F2 |
Cd/F3 |
Cd/NR |
Abelmoschus esculentus |
1.01 |
0.36 |
0.51 |
1.33 |
2.20 |
2.81 |
1.98 |
0.76 |
0.46 |
Benincasa hispida |
0.46 |
0.15 |
0.22 |
1.46 |
1.83 |
3.07 |
2.09 |
0.32 |
0.25 |
Capsicum annum |
0.17 |
0.05 |
0.18 |
0.12 |
0.35 |
3.40 |
0.94 |
1.42 |
0.49 |
Cucumis sativus |
0.62 |
0.27 |
0.25 |
0.89 |
1.41 |
2.30 |
2.48 |
0.70 |
0.44 |
Cucurbita moschata |
0.59 |
0.32 |
0.54 |
1.24 |
2.10 |
1.84 |
1.09 |
0.48 |
0.28 |
Lagenaria siceraria |
1.04 |
0.32 |
0.28 |
1.84 |
2.44 |
3.25 |
3.71 |
0.57 |
0.43 |
Luffa acutangula |
0.84 |
0.34 |
1.16 |
2.12 |
1.92 |
2.47 |
0.72 |
0.40 |
0.44 |
Momordica charantia L. |
0.76 |
0.18 |
0.37 |
0.89 |
1.44 |
4.22 |
2.05 |
0.85 |
0.53 |
Momordica sp. |
0.56 |
0.20 |
0.33 |
0.17 |
0.69 |
2.80 |
1.70 |
3.29 |
0.81 |
Solanum melongena |
1.32 |
0.56 |
0.80 |
1.47 |
2.83 |
2.36 |
1.65 |
0.90 |
0.47 |
Tricosanthes celebica |
0.40 |
0.13 |
0.51 |
0.79 |
1.43 |
3.08 |
0.78 |
0.51 |
0.28 |
Vigna sinensis |
0.53 |
0.39 |
0.8 |
1.28 |
2.47 |
1.36 |
0.66 |
0.41 |
0.21 |
Minimum |
0.17 |
0.05 |
0.18 |
0.12 |
0.35 |
1.36 |
0.66 |
0.32 |
0.21 |
Maximum |
1.32 |
0.56 |
1.16 |
2.12 |
2.83 |
4.22 |
3.71 |
3.29 |
0.81 |
Mean (12) |
0.69 |
0.27 |
0.50 |
1.13 |
1.76 |
2.75 |
1.65 |
0.88 |
0.42 |
Standard deviation |
0.32 |
0.14 |
0.29 |
0.60 |
0.73 |
0.75 |
0.90 |
0.81 |
0.16 |
Standard error |
0.09 |
0.04 |
0.09 |
0.17 |
0.21 |
0.22 |
0.26 |
0.24 |
0.05 |
Note: F1=‘easily, freely, leachable or exchangeable’ fraction; F2=‘acid-reducible’ fraction; F3=‘oxidisable-organic’ fraction; NR=non-resistant fraction (summation of F1, F2 and F3 fractions).
Table 5. The concentration (mean±SD, mg/kg dry weight) of Cd in the leafy vegetables, geochemical fractions of the habitat topsoils, and their transfer factors (Cd/F1, Cd/F3, Cd/F3, and Cd/NR) collected from three farming sites in Peninsular Malaysia
Leafy vegetables |
Cd |
CdF1 |
CdF2 |
CdF3 |
CdNR |
Cd/F1 |
Cd/F2 |
Cd/F3 |
Cd/NR |
Allium tuborosum |
0.74 |
0.30 |
0.54 |
0.87 |
1.71 |
2.47 |
1.37 |
0.85 |
0.43 |
Amaranthus gangeticus |
2.17 |
1.44 |
2.88 |
2.49 |
5.81 |
1.51 |
0.75 |
0.87 |
0.37 |
Amaranthus viridis |
2.08 |
1.21 |
1.09 |
2.68 |
4.98 |
1.72 |
1.91 |
0.78 |
0.42 |
Brassica rapa |
0.79 |
0.55 |
1.84 |
2.53 |
1.92 |
1.44 |
0.43 |
0.31 |
0.41 |
Ipomoea reptans |
0.91 |
0.36 |
0.56 |
1.07 |
1.99 |
2.53 |
1.63 |
0.85 |
0.46 |
Lactuca sativa |
1.03 |
0.58 |
0.96 |
0.57 |
2.11 |
1.78 |
1.07 |
1.81 |
0.49 |
Minimum |
0.74 |
0.30 |
0.54 |
0.57 |
1.71 |
1.44 |
0.43 |
0.31 |
0.37 |
Maximum |
2.17 |
1.44 |
2.88 |
2.68 |
5.81 |
2.53 |
1.91 |
1.81 |
0.49 |
Mean (6) |
1.29 |
0.74 |
1.31 |
1.70 |
3.09 |
1.91 |
1.19 |
0.91 |
0.43 |
Standard deviation |
0.66 |
0.47 |
0.90 |
0.96 |
1.81 |
0.48 |
0.55 |
0.49 |
0.04 |
Standard error |
0.27 |
0.19 |
0.37 |
0.39 |
0.74 |
0.19 |
0.23 |
0.20 |
0.02 |
Note: F1=‘easily, freely, leachable or exchangeable’ fraction; F2=‘acid-reducible’ fraction; F3=‘oxidisable-organic’ fraction; NR= non-resistant fraction (summation of F1, F2 and F3 fractions).
Table 6. The concentration (mean±SD, mg/kg dry weight) of Pb in the fruit vegetables, geochemical fractions of the habitat topsoils, and their transfer factors (Pb/F1, Pb/F3, Pb/F3, and Pb/NR) collected from three farming sites in Peninsular Malaysia
Fruit vegetables |
Pb |
PbF1 |
PbF2 |
PbF3 |
PbNR |
Pb/F1 |
Pb/F2 |
Pb/F3 |
Pb/NR |
Abelmoschus esculentus |
1.61 |
0.29 |
0.78 |
3.02 |
4.09 |
5.55 |
2.06 |
0.53 |
0.39 |
Benincasa hispida |
1.85 |
1.26 |
0.74 |
2.91 |
4.91 |
1.47 |
2.50 |
0.64 |
0.38 |
Capsicum annum |
0.62 |
0.09 |
1.39 |
1.12 |
2.6 |
6.89 |
0.45 |
0.55 |
0.24 |
Cucumis sativus |
0.74 |
0.42 |
0.31 |
1.35 |
2.08 |
1.76 |
2.39 |
0.55 |
0.36 |
Cucurbita moschata |
0.62 |
0.03 |
0.07 |
7.23 |
7.33 |
20.7 |
8.86 |
0.09 |
0.08 |
Lagenaria siceraria |
1.77 |
0.29 |
2.62 |
2.97 |
5.88 |
6.10 |
0.68 |
0.60 |
0.30 |
Luffa acutangula |
0.88 |
0.51 |
0.14 |
3.47 |
4.12 |
1.73 |
6.29 |
0.25 |
0.21 |
Momordica charantia |
1.79 |
0.25 |
0.71 |
2.80 |
3.76 |
7.16 |
2.52 |
0.64 |
0.48 |
Momordica charantia L. |
0.80 |
0.04 |
0.16 |
6.32 |
6.52 |
20.0 |
5.00 |
0.13 |
0.12 |
Solanum melongena |
1.54 |
0.49 |
1.36 |
1.21 |
3.06 |
3.14 |
1.13 |
1.27 |
0.50 |
Tricosanthes celebica |
1.36 |
0.24 |
1.68 |
1.27 |
3.19 |
5.67 |
0.81 |
1.07 |
0.43 |
Vigna sinesis |
0.74 |
0.04 |
1.48 |
1.03 |
2.55 |
18.5 |
0.50 |
0.72 |
0.29 |
Minimum |
0.62 |
0.03 |
0.07 |
1.03 |
2.08 |
1.47 |
0.45 |
0.09 |
0.08 |
Maximum |
1.85 |
1.26 |
2.62 |
7.23 |
7.33 |
20.7 |
8.86 |
1.27 |
0.50 |
Mean (12) |
1.19 |
0.33 |
0.95 |
2.89 |
4.17 |
8.22 |
2.77 |
0.59 |
0.32 |
Standard deviation |
0.50 |
0.34 |
0.77 |
2.03 |
1.67 |
7.23 |
2.64 |
0.34 |
0.13 |
Standard error |
0.14 |
0.10 |
0.22 |
0.59 |
0.48 |
2.09 |
0.76 |
0.10 |
0.04 |
Note: F1=‘easily, freely, leachable or exchangeable’ fraction; F2=‘acid-reducible’ fraction; F3=‘oxidisable-organic’ fraction; NR= non-resistant fraction (summation of F1, F2 and F3 fractions).
Table 7. The concentration (mean±SD, mg/kg dry weight) of Pb in the leafy vegetables, geochemical fractions of the habitat topsoils, and their transfer factors (Pb/F1, Pb/F3, Pb/F3, and Pb/NR) collected from three farming sites in Peninsular Malaysia
Leafy vegetables |
Pb |
PbF1 |
PbF2 |
PbF3 |
PbNR |
Pb/F1 |
Pb/F2 |
Pb/F3 |
Pb/NR |
Allium tuborosum |
1.42 |
0.42 |
1.58 |
1.31 |
3.31 |
3.38 |
0.9 |
1.08 |
0.43 |
Amaranthus tricolor |
2.74 |
0.30 |
0.72 |
8.94 |
9.96 |
9.13 |
3.81 |
0.31 |
0.28 |
Amaranthus viridis |
2.59 |
0.24 |
0.75 |
6.28 |
7.27 |
10.8 |
3.45 |
0.41 |
0.36 |
Brassica rapa |
1.26 |
0.37 |
1.05 |
24.0 |
25.4 |
3.41 |
1.20 |
0.05 |
0.05 |
Ipomoea reptans |
1.56 |
0.23 |
0.59 |
12.3 |
13.1 |
6.78 |
2.64 |
0.13 |
0.12 |
Lactuca sativa |
1.23 |
0.36 |
0.93 |
27.5 |
28.8 |
3.42 |
1.32 |
0.04 |
0.04 |
Minimum |
1.23 |
0.23 |
0.59 |
1.31 |
3.31 |
3.38 |
0.90 |
0.04 |
0.04 |
Maximum |
2.74 |
0.42 |
1.58 |
27.5 |
28.8 |
10.8 |
3.81 |
1.08 |
0.43 |
Mean (6) |
1.80 |
0.32 |
0.94 |
13.4 |
14.6 |
6.15 |
2.22 |
0.34 |
0.21 |
Standard deviation |
0.68 |
0.08 |
0.35 |
10.3 |
10.2 |
3.27 |
1.25 |
0.39 |
0.17 |
Standard error |
0.28 |
0.03 |
0.14 |
4.20 |
4.18 |
1.33 |
0.51 |
0.16 |
0.07 |
Note: F1=‘easily, freely, leachable or exchangeable’ fraction; F2=‘acid-reducible’ fraction; F3=‘oxidisable-organic’ fraction; NR= non-resistant fraction (summation of F1, F2 and F3 fractions).
Table 8. The mean concentrations (mg/kg wet weight) of fruit vegetables in the vegetables grown on reclaimed tidal flat soils in the Pearl River Estuary (China).
Fruit vegetables |
Species |
Cd |
Pb |
Eggplant |
Solanum melongena |
0.039 |
0.034 |
Tomato |
Lycopersicon esculentum |
0.020 |
0.023 |
Cucumber |
Cucumis sativus L. |
0.009 |
0.023 |
Suakwa towel gourd |
Luffa cylindrical (Linn.) Roem. |
0.013 |
0.022 |
Bitter gourd |
Momordica charantia |
0.019 |
0.015 |
Green cowpea |
Vigna unguiculata (Linn.) |
0.005 |
0.075 |
|
Minimum |
0.005 |
0.015 |
|
Maximum |
0.039 |
0.075 |
|
Mean |
0.018 |
0.032 |
|
Standard deviation |
0.012 |
0.022 |
|
Standard error |
0.005 |
0.009 |
Table 9. The mean concentrations (mg/kg wet weight) of leafy vegetables grown on reclaimed tidal flat soils in the Pearl River Estuary (China)
Leafy vegetables |
Species |
Cd |
Pb |
Cabbage |
Brasssica oleracea L. var capitata L |
0.048 |
0.074 |
Chinese lactuca |
Lactuca sativa L. var. asparagina |
0.060 |
0.128 |
Pakchoi |
Brassica chinensis |
0.037 |
0.078 |
Chinese flowering cabbage |
Brassica rapa chinensis |
0.035 |
0.106 |
Romaine lettuce |
Lactuca sativa L. var. longifolia |
0.053 |
0.057 |
Edible amaranth |
Amaranthus mangostanus L. |
0.078 |
0.132 |
Water spinach |
Ipomaea aquatica Forssk |
0.035 |
0.021 |
Leaf mustard |
Brassica juncea Coss |
0.056 |
0.047 |
|
Minimum |
0.035 |
0.021 |
|
Maximum |
0.078 |
0.132 |
|
Mean |
0.050 |
0.080 |
|
Standard deviation |
0.015 |
0.039 |
|
Standard error |
0.005 |
0.014 |
Yang, et al. [25] reported that the Cd concentrations (mg/kg ww) in edible vegetables collected from the six selected greenhouse vegetable bases from eastern China were consistently higher in the leafy vegetables (0.009-0.09) than those (0.002-0.017) in the fruit vegetables in all the six bases, and these levels were below the maximum permissible concentration for Cd (0.05 mg/kg ww) for edible parts of vegetables established by China [26].
Yang, et al. [25] reported that the Pb concentrations (mg/kg ww) in edible vegetables collected from the six selected greenhouse vegetable bases from eastern China were 0.014-0.092 in the leafy vegetables and 0.006-0.175 in the fruit vegetables, where four out of six bases had higher Pb levels in the leafy than the fruit vegetables. These levels were below the maximum permissible concentration for Pb (0.20 mg/kg ww) for edible parts of vegetables established by China [26].
Hu, et al. [27] reported the mean concentrations (mg/kg ww) of Cd and Pb in the four species of leafy vegetables (Cd: 0.013; Pb: 0.022) are higher than those in the four species of fruit vegetables (Cd: 0.005; Pb: 0.019). Fan, et al. [28] reported that the concentrations (mg/kg ww) of Cd and Pb in greenhouse vegetables were 0.02 and 0.26, respectively for fruit vegetables while they were 0.03 and 0.74 for leafy vegetables. These Cd levels were lower than those recommended for limits in Chinese vegetables [29] namely fruit and leafy vegetables as 0.05 and 0.20 mg/kg ww for Cd, respectively. However, the Pb levels were higher those recommended for limits in Chinese vegetables [29] namely fruit and leafy vegetables as 0.10 and 0.30 mg/kg ww for Pb, respectively.
Similarly, Chary, et al. [30] reported the higher enrichment factor for heavy metals in leafy vegetables. According to Yang, et al. [31], translocation of heavy metal ions from soil to plants is regulated by the root cell wall, the ion transmembrane transport in the endoderm cytoplasmic membrane, and the water transport in the xylem vessel. The latter is further controlled by transpiration [32].
In general, the trend of metal transfer in different vegetable types was ordered as leafy > fruit vegetables. The higher concentrations of Cd and Pb in leafy than those in the fruit vegetables implied that the leafy vegetables had higher risk for the accumulation of both metals. The higher levels of heavy metals in the leafy vegetables agreed with previous findings in the literature [33,34]. This indicated that leafy vegetable has higher transportation rates than other vegetable types [35]. This might be due to the more barriers preventing heavy metals’ transmission from soil to fruits than those to leaves [36].
Transfer factor
Between the fruit and leafy vegetables, the levels of Cd and Pb are significantly (P< 0.05) higher in the leafy vegetables than those in the fruit vegetables (Tables 4-7). However, the TF values of leafy vegetables in Cd and Pb (Tables 4–7) were lower than those in the fruit vegetables.
Based on the Cd TF for the fruit vegetables (Table 4), they range from 1.36-4.22 for Cd/F1, 0.66-3.71 for Cd/F2, 0.32-3.29 for Cd/F3 and 0.21-0.81 for Cd/NR. Based on the Cd TF for the leafy vegetables (Table 5), they range from 1.44-2.53 for Cd/F1, 0.43-1.91 for Cd/F2, 0.31-1.81 for Cd/F3 and 0.37-0.49 for Cd/NR.
Based on the Pb TF for the fruit vegetables (Table 6), they range from 1.47-20.67 for Pb/F1, 0.45-8.86 for Pb/F2, 0.09-1.27 for Pb/F3 and 0.08-0.50 for Pb/NR. Based on the Pb TF for the leafy vegetables (Table 7), they range from 3.38-10.79 for Pb/F1, 0.90-3.81 for Pb/F2, 0.04-1.08 for Pb/F3 and 0.04-0.43 for Pb/NR.
Lavado [37] reported that the TF of Cd in vegetable was higher than those of any other metals examined in all vegetables. This indicated that there is a higher human health risk of Cd due to its toxicity and mobility from soil to vegetables. Since TF values for Pb/F1 and Pb/F2 in fruits and leafy vegetables were observed to be higher than those for Cd in the present study, Pb is assumed to have a higher bioavailability to the vegetables via F1 and F2 fractions of the corresponding topsoils.
This indicated that Pb is more easily transferable to fruit and leafy vegetables, while transfer of Cd from F1 and F2 of the habitat topsoils into the edible parts of vegetables faced much more resistance [11]. According to Adamo, et al. [38], the TF variations of metals in different types of vegetables could be connected to each vegetable's absorption capability, soil properties and soil nutrient management.
Based on the mean values in Tables 4 to 7, the TF values of Cd/F1 and Pb/F1 in fruit vegetables are higher than those in the leafy vegetables. This implies that Cd and Pb can be easily accumulated in the fruit vegetables from the F1 fraction of the topsoils.
Metal levels in the topsoils
Based on Tables 4 and 5, the Cd concentrations (mg/kg dw) in the F1, F2, F3 and NR of the habitat topsoils range from 0.05 – 1.44, 0.18- 2.88, 0.12- 2.68, and 0.35- 5.81, respectively. Based on Tables 6 and 7, the Pb concentrations (mg/kg dw) in the F1, F2, F3 and NR of the habitat topsoils range from 0.03- 1.26, 0.07- 2.62, 1.03- 27.5, and 2.08- 28.8, respectively.
Relationships of metals between vegetables and geochemical fractions of the topsoils
The relationships of Cd between the vegetables and their habitat topsoils (four geochemical fractions: F1, F2, F3 and NR, are presented in Figure 2. It is found that the Cd levels in the vegetables are highly correlated with the F1 (R=0.94), F2 (R=0.68), F3 (R=0.69), and NR (R=0.93) fractions of the habitat topsoils. This indicated that Cd geochemical fractions (F1, F2, F3 and NR) in the habitat topsoils are considered readily and potentially bioavailable to the vegetables [39]. Therefore, continuous root uptake of Cd from habitat topsoils to vegetables can be achieved because of their good correlations of Cd between the vegetables and habitat topsoils.
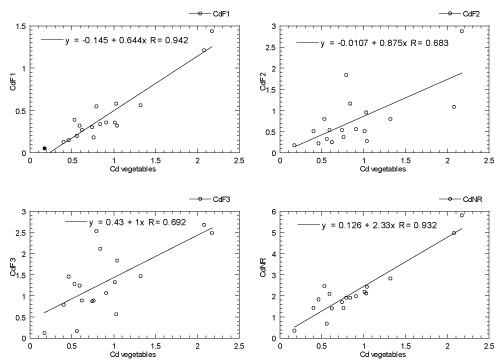
Figure 2. Relationships of Cd between the vegetables and their habitat topsoils (four geochemical fractions: F1, F2, F3 and NR). Note: F1=easily, freely, leachable or xchangeable fraction; F2=acid-reducible fraction; F3=Oxidisable-organic fraction; NR=Non-resistant fraction (summation of F1, F2 and F3 fractions).
The relationships of Pb between the vegetables and their habitat topsoils (four geochemical fractions: F1, F2, F3 and NR, are presented in Figure 3. It is found that the Pb levels in the vegetables are very weakly (low) correlated with the F1, F2, F3 and NR (R=0.06 to 0.29) fractions of the habitat topsoils. Practically, there is no clear correlations for other pairwises.
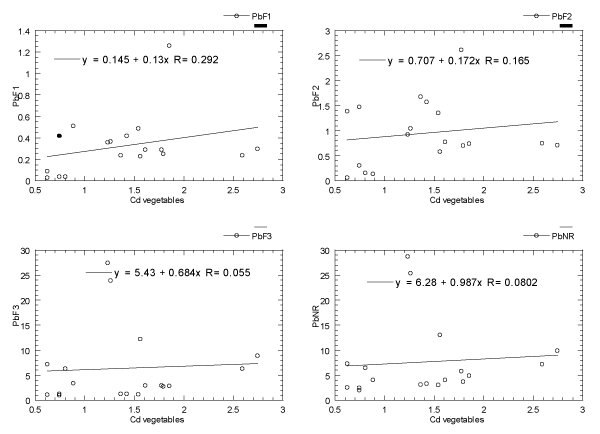
Figure 3. Relationships of Pb between the vegetables and their habitat topsoils (four geochemical fractions: F1, F2, F3 and NR). Note: F1=easily, freely, leachable or exchangeable fraction; F2=acid-reducible fraction; F3=Oxidisable-organic fraction; NR=Non-resistant fraction (summation of F1, F2 and F3 fractions).
These strong relationships are not found for Pb. Besides the root uptake of Pb to the leaves, atmospheric deposition could influence the bioavailability and contamination of Pb in local vegetables [14]. Xu, et al. [40] concluded that the soil metal bioavailability to the vegetables was generally dependent on the particular metal and vegetable species. However, Liu, et al. [41] found that the metal concentrations in vegetables and corresponding soils were poorly correlated.
Fan, et al. [28] studied the correlation of heavy metal levels between the greenhouse vegetables and soil general properties (including the geochemical fractions). They found that the concentrations of Pb in greenhouse vegetables were significantly correlated with concentrations of different forms of heavy metals in greenhouse soil, indicating that Pb in vegetables could be predicted by concentrations of different forms of heavy metals in soils.
As shown in Table 4, the correlations of Cd and Pb between vegetables and habitat topsoils varied greatly for different vegetable types. This agrees to those reported by Fan, et al. [28] and Yang, et al. [33]. This was probably due to different absorption mechanism of metals in different types of vegetables [42]. There was no significantly correlation of Pb levels between vegetables and habitat topsoils. It was probably due to some other factors such as cation exchange capacity, which could have influenced the Pb availability in the habitat topsoils.
Health risk assessments
The values of EDI and THQ of Cd and Pb in the 18 vegetables for adults and children from the present study, are presented in Table 10. From Table 10, it is found that the THQ for Cd in the Amaranthus viridis and A. tricolor were higher than 1.00, indicating the potential non-carcinogenic risk of Cd to the consumers for both adults and children. Overall statistics of EDI and THQ values for adults and children from the present study are given in Table 11. The EDI values of Cd for adults and children range from 0.10-1.30, and 0.11-1.49, respectively. The EDI values of Pb for adults and children range from 0.26-1.61, and 0.30-1.85, respectively. The THQ values of Cd for adults and children range from 0.10-1.30, and 0.11-1.49, respectively. The THQ values of Pb for adults and children range from 0.06-0.40, and 0.07-0.46, respectively. Therefore, except for the two vegetables above, all the THQ values in the other 16 vegetables for Cd and Pb in both adult and children are all below 1.0. This indicates there is no non-carcinogenic risk of Cd and Pb via the consumption of the 16 vegetables from the present study.
Table 10. Estimated daily intake (EDI) and target hazard quotient (THQ) values of Cd and Pb in the 18 vegetables for adults and children from the present study
Vegetables |
|
EDI |
THQ |
| |
|
Cd |
Pb |
Cd |
Pb |
Momordica charantia (n=6) |
Adults |
0.24 |
0.78 |
0.24 |
0.20 |
| |
Children |
0.28 |
0.90 |
0.28 |
0.22 |
Abelmoschus esculentus (n=12) |
Adults |
0.69 |
1.10 |
0.69 |
0.27 |
| |
Children |
0.79 |
1.26 |
0.79 |
0.31 |
Cucumis sativus (n=7) |
Adults |
0.23 |
0.27 |
0.23 |
0.07 |
| |
Children |
0.26 |
0.32 |
0.26 |
0.08 |
Amaranthus viridis (n=16) |
Adults |
1.30 |
1.61 |
1.30 |
0.40 |
| |
Children |
1.49 |
1.85 |
1.49 |
0.46 |
Amaranthus tricolor (n=16) |
Adults |
1.06 |
1.34 |
1.06 |
0.34 |
| |
Children |
1.22 |
1.54 |
1.22 |
0.39 |
Benincasa hispida (n=6) |
Adults |
0.21 |
0.83 |
0.21 |
0.21 |
| |
Children |
0.24 |
0.95 |
0.24 |
0.24 |
Capsicum annum (n=12) |
Adults |
0.10 |
0.35 |
0.10 |
0.09 |
| |
Children |
0.11 |
0.40 |
0.11 |
0.10 |
Lactuca sativa (n=6) |
Adults |
0.43 |
0.51 |
0.43 |
0.13 |
| |
Children |
0.49 |
0.59 |
0.49 |
0.15 |
Ipomoea reptans (n=18) |
Adults |
0.56 |
0.96 |
0.56 |
0.24 |
| |
Children |
0.65 |
1.11 |
0.65 |
0.28 |
Solanum melongena (n=6) |
Adults |
0.62 |
0.72 |
0.62 |
0.18 |
| |
Children |
0.71 |
0.83 |
0.71 |
0.21 |
Brassica rapa (n=8) |
Adults |
0.52 |
0.83 |
0.52 |
0.21 |
| |
Children |
0.60 |
0.95 |
0.60 |
0.24 |
Allium tuborosum (n=22) |
Adults |
0.38 |
0.73 |
0.38 |
0.18 |
| |
Children |
0.44 |
0.84 |
0.44 |
0.21 |
Momordica charantia L. (n=6) |
Adults |
0.35 |
0.37 |
0.35 |
0.09 |
| |
Children |
0.40 |
0.42 |
0.40 |
0.11 |
Vigna sinesis (n=16) |
Adults |
0.19 |
0.26 |
0.19 |
0.06 |
| |
Children |
0.21 |
0.30 |
0.21 |
0.07 |
Lagenaria siceraria (n=6) |
Adults |
0.67 |
1.13 |
0.67 |
0.28 |
| |
Children |
0.76 |
1.30 |
0.76 |
0.33 |
Luffa acutangula (n=6) |
Adults |
0.28 |
0.29 |
0.28 |
0.07 |
| |
Children |
0.32 |
0.33 |
0.32 |
0.08 |
Tricosanthes celebica (n=6) |
Adults |
0.13 |
0.44 |
0.13 |
0.11 |
| |
Children |
0.15 |
0.51 |
0.15 |
0.13 |
Cucurbita moschata (n=5) |
Adults |
0.30 |
0.32 |
0.30 |
0.08 |
| |
Children |
0.35 |
0.36 |
0.35 |
0.09 |
Note: Daily consumption for adults is 345 and 232 for children (g/day) and average body weight 55.90kg and 32.70kg for adults and children respectively
Table 11. Overall statistics of estimated daily intake (EDI) and target hazard quotient (THQ) values for adults and children from the present study
|
EDI |
|
THQ |
|
Adults |
Cd |
Pb |
Cd |
Pb |
Minimum |
0.10 |
0.26 |
0.10 |
0.06 |
Maximum |
1.30 |
1.61 |
1.30 |
0.40 |
Mean |
0.46 |
0.71 |
0.46 |
0.18 |
Standard deviation |
0.32 |
0.40 |
0.32 |
0.10 |
Standard error |
0.08 |
0.09 |
0.08 |
0.02 |
Children |
Cd |
Pb |
Cd |
Pb |
Minimum |
0.11 |
0.30 |
0.11 |
0.07 |
Maximum |
1.49 |
1.85 | 2021 Copyright OAT. All rights reserv
1.49 |
0.46 |
Mean |
0.53 |
0.82 |
0.53 |
0.21 |
Standard deviation |
0.37 |
0.46 |
0.37 |
0.12 |
Standard error |
0.09 |
0.11 |
0.09 |
0.03 |
From the present study based on 12 fruit vegetables and 6 leafy vegetables, the levels of Cd and Pb are all significantly (P<0.05) higher in the leafy vegetables than those in the fruit vegetables. It is found that the Cd in the vegetables are highly correlated with the Cd geochemical fractions (F1, F2, F3 and NR) in the habitat topsoils. These positive relationships indicated the potential of edible vegetables as good biomonitors of Cd pollution in the habitat topsoils. For the health risk assessment, al the THQ values for Cd and Pb in both adult and children are all below 1.00, except for the THQ for Cd in the Amaranthus viridis and A. tricolor which were higher than 1.00, indicating the potential non-carcinogenic risk of Cd to the consumers for both adults and children. Hence, the routine monitoring and management of the vegetable farms is recommended and necessary.
The authors wish to acknowledge the partial financial support provided through the Fundamental Research Grant Scheme (FRGS), [Vote no.: 5524953], by Ministry of Higher Education, Malaysia.
The authors declare that there are no conflicts of interest.
- Jolly YN, Islam A, Akbar S (2013) Transfer of metals from soil to vegetables and possible health risk assessment. Springer Plus 2: 385.
- Eliku T, Leta S (2017) Heavy metals bioconcentration from soil to vegetables and appraisal of health risk in Koka and Wonji farms, Ethiopia. Environ Sci Pollut Res 24: 11807–11815.
- Islam MS, Ahmed MK, Habibullah-Al-Mamun M (2016) Apportionment of heavy metals in soil and vegetables and associated health risks assessment. Stochastic Environ Res Risk Assess 30: 365–377.
- Chang CY, Yu HY, Chen JJ, Li FB, Zhang HH, et al. (2014) Accumulation of heavy metals in leaf vegetables from agricultural soils and associated potential health risks in the Pearl River Delta, South China. Environ Monit Assess 186: 1547–1560.
- Garg VK, Yadav P, Mor S, Singh B, Pulhani V (2014) Heavy metals bioconcentration from soil to vegetables and assessment of health risk caused by their ingestion. Biol Trace Element Res 157: 256–265.
- Lion GN, Olowoyo JO (2013) Population health risk due to dietary intake of toxic heavy metals from Spinacia oleracea harvested from soils collected in and around Tshwane, South Africa. South Afr J Bot 88: 178–182.
- Douay F, Pelfrêne A, Planque J, Fourrier H, Richard A, et al. (2013) Assessment of potential health risk for inhabitants living near a former lead smelter. Part 1: Metal concentrations in soils, agricultural crops, and homegrown vegetables. Environ Monit Assess 185: 3665–3680.
- Lawal NS, Agbo O, Usman A (2017) Health risk assessment of heavy metals in soil, irrigation water and vegetables grown around Kubanni River, Nigeria. J Phys Sci 28: 49–59.
- Ro M, Mcdonald LM (2015) Metal uptake in plants and health risk assessments in metal-contaminated smelter soils. Land Degrad Develop 26: 785–792.
- Xue ZJ, Liu SQ, Liu YL, Yan YL (2012) Health risk assessment of heavy metals for edible parts of vegetables grown in sewage-irrigated soils in suburbs of Baoding City, China. Environ Monit Assess 184: 3503–3513.
- Khan S, Cao Q, Zheng YM, Huang YZ, Zhu YG (2008) Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ Pollut 152: 686–692.
- Bian B, Wu H, Lv L, Fan Y, Lu H (2015) Health risk assessment of metals in food crops and related soils amended with biogas slurry in Taihu Basin: perspective from field experiment. Environ Sci Pollut Res 22: 14358–14366.
- Zhang C, Wang Y, Zhang Z, Wang D, Luo C, et al. (2015) Health risk assessment of heavy metals and as in vegetable and soil system in Chongqing, Southwest of China. J Resid Sci Tech 12: 231–240.
- Islam MS, Ahmed MK, Habibullah-Al-Mamun M, Masunaga S (2014) Trace metals in soil and vegetables and associated health risk assessment. Environ Monit Assess 186: 8727-8739. [Crossref]
- Zhang H, Huang B, Dong L, Hu W, Akhtar MS, et al. (2017) Accumulation, sources and health risks of trace metals in elevated geochemical background soils used for greenhouse vegetable production in southwestern China. Ecotox Environ Saf 137: 233–239.
- Chin HF, Yap EE (1999) Malaysian vegetables in colour: A complete guide. Kuala Lumpur: Tropical Press.
- Prohens J, Nuez F (2008a) Vegetables I: Asteraceae, Brassicaceae, Chenopodicaceae, and Cucurbitaceae. New York: Springer.
- Prohens J, Nuez F (2008b) Vegetables II: Fabaceae, Liliaceae, Solanaceae, and Umbelliferae. New York: Springer.
- Badri MA, Aston SR (1983) Observation on heavy metal geochemical associations in polluted and non-polluted estuarine sediments. Environ Pollut Ser B6:181–193.
- Wang X, Sato T, Xing B, Tao S (2005) Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci Tot Environ 350: 28–37.
- USEPA (United States Environmental Protection Agency). 2000. Risk-based Concentration Table. United State Environmental Protection Agency, Washington, DC.
- IRIS (Integrated Risk Information System) (2000) Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. US Environmental Protection Agency. http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=22567#Download (accessed 27.05.15).
- FAO/WHO (2013) Guidelines for the Safe Use of Wastewater and food stuff; Volume 2: No1 14, pp 988. Wastewater Use in Agriculture, World Health Organization, Geneva.
- Li Q, Chen Y, Fu H, Cui Z, Shi L, et al. (2012) Health risk of heavy metals in food crops grown on reclaimed tidal flat soil in the Pearl River Estuary, China. J Hazard Mater 227–228:148–154.
- Yang LQ, Huang B, Hu WY, Chen Y, Mao MC, et al. (2014) The impact of greenhouse vegetable farming duration and soil types on phytoavailability of heavy metals and their health risk in eastern China. Chemosphere 103: 121–130.
- AQSIQ (2001) Safety Qualification for Agricultural Product – Safety Requirements for Non-Environmental Pollution Vegetable (GB18406.1-2001). General Administration of Quality Supervision, Inspection and Quarantine of China, Beijing, China.
- Hu W, Huang B, Tian K, Holm PE, Zhang Y (2017) Heavy metals in intensive greenhouse vegetable production systems along Yellow Sea of China: Levels, transfer and health risk. Chemosphere 167: 82–90.
- Fan Y, Li H, Xue Z, Zhang Q, Cheng F (2017) Accumulation characteristics and potential risk of heavy metals insoil-vegetable system under greenhouse cultivation condition in Northern China. Ecol Eng 102: 367–373.
- AQSIQ (2012) Limits in Food Contaminants (GB2762-2012). General Administration of Quality Supervision, Inspection and Quarantine of China, Beijing, China.
- Chary NS, Kamala CT, Raj DSS (2008) Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotox Environ Saf 69: 513–524.
- Yang X, Feng Y, He Z, Stoffella PJ (2005) Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. J Trace Elem Med Biol 18: 339-353. [Crossref]
- Tani FH, Barrington S (2005) Zinc and copper uptake by plants under two transpiration rates. Part I. Wheat (Triticum aestivum L.). Environ Pollut 138: 538–547.
- Yang H, Liang QL, Zhao L, Zhu C (2012) The cumulative effect on heavy metal of seven kinds of vegetable crops and effects on heavy metal absorption of intercropping Kummerowia striata. J Soil Water Conserv 26: 209–214.
- Peris M, Micó C, Recatalá L, Sánchez R, Sánchez J (2007) Heavy metal contents in horticultural crops of a representative area of the European Mediterranean region. Sci Tot Environ 378: 42–48.
- Xu L, Lu AX, Wang JH, Ma ZH, Pan LG, et al. (2015) Accumulation status, sources and phytoavailability of metals in greenhouse vegetable production systems in Beijing, China. Ecotox Environ Saf 122: 214–220.
- Sun FF, Wang FH, Wang X, He W, Wen DA, et al. (2013) Soil threshold values of total and available cadmium for vegetable growing based on field data in Guangdong province, South China. J Sci Food Agr 93: 1967–1973.
- Lavado RS (2006) Concentration of potentially toxic elements in field crops grown near and far from cities of the Pampas (Argentina). J Environ Manage 80: 116–119.
- Adamo P, Lavazzo P, Albanese S, Agrelli D, De Vivo B, et al. (2014) Bioavailability and soil-to-plant transfer factors as indicators of potentially toxic element contamination in agricultural soils. Sci Tot Environ 500: 11–22.
- Liu HY, Probst A, Liao BH (2005) Metal contamination of soils and crops affected by the Chenzhou lead zinc mine spill (Hunan, China). Sci Total Environ 339: 153–166.
- Xu D, Zhou P, Zhan J, Gao Y, Dou C, et al. (2013) Assessment of trace metal bioavailability in garden soils and health risks via consumption of vegetables in the vicinity of Tongling mining area, China. Ecotox Environ Saf 90: 103–111.
- Liu X, Song Q, Tang Y, Li W, Xu J, et al. (2013) Human health risk assessment of heavy metals in soil-vegetable system: a multi-medium analysis. Sci Total Environ 463-464: 530-40. [Crossref]
- Khan A, Khan S, Khan MA, Qamar Z, Waqas M (2015) The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review. Environ Sci Pollut Res 22: 13772–13799.



