Various oxygen species (such as hydroxyl-OH, peroxyl-ROO radicals), formed in human tissue cells by many endogenous and exogenous causes, cause extensive oxidative damage which according to a large body of scientific evidence links to initiation and development of aging, cardiovascular and other human diseases. This review paper first provides an overview of free radicals as initiators of oxidative stress with focus on LDL and DNA oxidative changes that are respectively associated with atherosclerosis and carcinogenecis. Based on common bio-indicators used to monitor LDL and DNA harmful oxidative processes, the paper provides a literature overview of the protective role that certain vitamins can exert (individually or in mixtures) against the health diseases relevant to oxidative stress.
DNA damage, oxidized LDL, provitamin E, tocopherols, ascorbic acid
DNA-DeoxyriboNucleic Acid
4-HNE (4-hydroxynonenal)
LDL (low-density lipoproteins)
MDA (Malondialdehyde)
8-OH-dG (8-hydroxy-2’-deoxyguanosine )
PUFAs (polyunsaturated fatty acids)
ROS (Reactive oxygen species)
TBARS (thiobarbituric acid reacting substances).
Reactive oxygen species
Reactive oxygen species (ROS) in vivo possess important roles in living organisms through their beneficial and detrimental effects [1,2]. Free radicals are formed in tissue cells by many endogenous and exogenous causes [3]. They are produced either (i) from normal cell metabolisms in situ (normal aerobic respiration i.e. mitochondria, stimulated polymorphulated leucocytes and macrophages) [4] or (ii) from external sources (pollution, cigarette smoke, radiation, medication) [5]. Oxygen free radicals (such as hydroxyl radicals; superoxide radicals and other active oxygen species such as singlet oxygen) adversely alter lipids, proteins, and DNA and trigger a number of human diseases [6]. A role of lipid peroxidation and oxidative stress in the association between thyroid diseases and breast cancer has been claimed by Dominguez and Castelao (2008) [7]. The ability of ROS to structurally modify cellular components, gene expression and protein production has led to the implication of their involvement in a variety of health diseases [8]. More specifically, ROS generate DNA oxidative damage and adversely affect biological membranes (e.g. LDL) of which pathological consequence, including cancer and cardiovascular diseases are well established [9]. Several of the most common in vivo ROS are shown in (Table 1), together with their major sources and target of damage [10].
Table 1. Range of the most common in vivo reactive oxygen species (Kiokias 2002) [10].
Radical |
Formation |
Target or type of damage |
Hydroxyl (OH·) |
Production from O2 or H2O2 in presence of transition metals |
Damage to DNA
and proteins
|
Superoxide (O2·-) |
Produced enzymatically by e- addition to O2 by SOD, or non-enzymatically from H2O2 |
Attack biological membranes,
sugars (oxidative damage)
|
Peroxyl or alkoxy (ROO· , RO·) |
Formation through the breakdown of organic peroxides |
Lipid peroxidation reaction and
initiation of atherosclerosis
|
Singlet oxygen (1O2) |
Photochemical activation of 1O2 in presence of sensitizers |
Lipid photoxidative damage skin-damage, carcinogenesis
|
Nitric oxide (NO*) |
In vivo production from L-arginine |
Pathogenetic in overproduction
|
LDL oxidative modification and atherosclerosis
Over the last few years, extensive clinical and epidemiologic research evidence has been gathered on the role of oxidized LDL (low-density lipoproteins) in the progression of atherosclerosis, as a risk factor for the development of coronary artery disease [11,12]. Atherosclerosis is a progressive disease of the arterial tree that involves deposition of lipid, mostly oxidized LDL, in the arterial intima leading finally to a thickening of the arterial wall and reduced luminal blood flow [13]. Oxidative modification of LDL, a lipid peroxidation reaction driven by free radicals is therefore a key step in the early stages of atherosclerosis [14,15]. The relationship between circulating ox-LDL and subclinical atherosclerosis has been recently explored and confirmed in one case control study performed by Fang, et al. (2011) [16] and two community-based cohort studies [17,18].
DNA oxidative damage and carcinogenesis
The development of cancer is a multistep process that involves a complex series of cellular and molecular changes mediated by a diversity of endogenous and exogenous stimuli [19]. It has recently become apparent that ROS generation from mitochondria first as the cellular response to oxidative stress and DNA damage is closely linked to carcinogenesis [20]. A body of research has in depth investigated into mechanisms of oxidative DNA damage trying to clarify how the components of the repair pathways may influence the cancer transformation. [21,22]. DNA damage can take many forms, ranging from specifically oxidised purine and pyrimidine bases (more than 20 such oxidative lesions have been identified) to gross DNA changes such as strand breaks, sister chromatid exchange, and the formation of micronuclei [23]. According to one of the proposed oxidative mechanisms, hydrogen peroxide can cause DNA strand breakage, by generation of the hydroxyl radical (OH·) close to the DNA molecule, via the Fenton reaction.
H2O2 + Fe2+ → OH· + OH- + Fe3+
This may result in DNA instability, mutagenesis and ultimately carcinogenesis [24]. Specific DNA oxidation products accumulate depending on the ROS involved, its rate of production, and the cell’s ability to protect or repair its DNA insult. Research efforts are intense to further elucidate DNA base excision repair, the primary mechanism to protect cells from genotoxicity caused by ROS [25].
A number of oxidative biomarkers have linked oxidative stress and the development of health diseases. An overview of the most commonly used is given below:
Estimation of plasma levels of oxidized LDL via formation of conjugated dienes (c.d)
The formation of conjugated dienes is generally accepted as evidence of lipid peroxidation and is due to re-arrangement of the double bonds which in presence of oxygen can form hydroperoxides [26]. A convenient and very frequently used method for monitoring the level of plasma oxidized LDL is the process of copper-induced LDL oxidation continuously through the spectrophotometric measurement of diene absorption at 233 nm [27]. The chronology of LDL oxidation by Cu2+ ions can be divided into three consecutive time phases: lag phase, propagation phase and decomposition phase [28]. During the lag phase, LDL becomes progressively depleted of its endogenous antioxidants, with α-tocopherol as the first to be lost and β-carotene as the last to remain. Depleted of its antioxidants the LDL particle enters the propagation phase in which the polyunsaturated fatty acids (PUFAs) are rapidly converted to conjugated lipid hydroperoxides indicated by the increase in absorbance at 233 nm [29]. Secondary reactions of LDL oxidation leading to aldehydes (malondialdehyde, hexanal, 4-hydroxynonenal etc.), are accelerated by transition metal ions, such as Fe2+, which may catalyse decomposition of lipid hydroperoxides to alkoxyl radicals in a Fenton-type reaction [30]:
LOOH + Fe2+ → LO· + OH- + Fe3+
Biomarkers of DNA oxidative damage
Among many kinds of DNA damage that occur due to ROS and that increase cancer risks, the most studied base damage without a question is 8-oxoguanine (8-oxoG) [31]. Generation of (8-oxoGua) in DNA is a mutagenic and potentially carcinogenic event since the oxidised base has altered hydrogen bonding or “code specificity”: preferentially forming base pairs with adenine rather than cytosine [32]. The modified base 8-oxo-guanine, its deoxy-nucleoside derivative 8-OH-dG (8-hydroxy-2’-deoxyguanosine) and 8-oxo-adenine (Figure 1) have been used as useful markers of oxidative DNA damage [9,10]. The effect of natural antioxidant vitamins against DNA oxidative damage will be extensively discussed in the section 3.
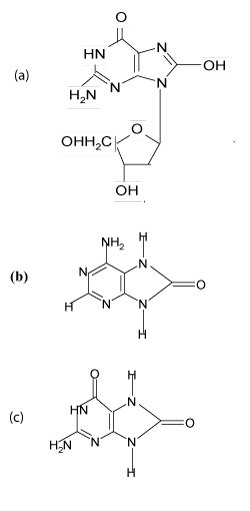
Figure 1. Selective products of DNA oxidative damage commonly used as bio-indicators of oxidative stress: (a) 8-OH-deoxyguanosine (b) 8-oxo adenine (c) 8-oxo-guanine (Kiokias, 2002) [10].
The Japan Institute for the Control of Aging [33] has developed an in vitro enzyme-linked immunosorbent assay (ELISA) for quantitative measurement of the oxidative DNA adduct 8-OH-dG in tissue or urine samples. As creatinine levels in urine are a measure of the concentration of the fluid, they co-vary with 8-OH-dG and determination of urinary creatinine is important in expressing the level of this DNA oxidative product with this method [27].
A method known as the COMET assay is an alternative sensitive and valuable technique that allows the detection of intercellular differences in DNA [34]. It allows the determination of oxidised DNA bases by making use of repair enzymes to introduce strand breaks at sites where oxidised bases are present [35]. The sensitivity and specificity of the COMET assay is greatly enhanced if the nucleoids are incubated with bacterial repair endonucleases that recognize specific kinds of damage in the DNA and convert lesions to DNA breaks, increasing the amount of DNA in the comet tail [36].
Lipid hydroperoxides
Lipid hydroperoxides are the primary products of lipid peroxidation and can be measured in several different ways [10,37]:
- by HPLC coupled with chemiluminescence. This method is very sensitive and
interference by biological antioxidants is avoided.
- by GC-MS after reduction to alcohols
- by the iodometric method, which is a sensitive method involving the reaction of
hydroperoxides with iodide in acid to form iodine as shown below:
ROOH + 2H +2I- → R-OH +H2O +I2
- by the FOX (Ferric oxidation of Xylenol). This a highly reproducible method for biological samples, based on the fact that hydroperoxides oxidise ferrous ion
(Fe++) to ferric ion (Fe+++) that can be detected by use of ferric sensitive dyes [38].
Measurement of thiobarbituric acid reacting substances (TBARS)
The TBARS test is still the most frequently used test to assess secondary products of lipid peroxidation [39]. Among these substances, malondialdehyde (MDA), which is formed in vivo from trienes via unsaturated peroxides, reflects unsaturated fatty acid composition as much as extent of lipid peroxidation [40]. MDA reacts with TBA reagent (in a 1:2 molar ratio) on heating under acidic conditions to give a red chromophore, which is measured by UV at 532 nm or by fluorescence at 553 nm [41] TBARS test is easy to perform and inexpensive. However, it lacks specificity as chromogens are formed with many aldehydes other than MDA, and with carbohydrates, amino acids etc [41].
Determination of Isoprostanes
Isoprostanes are prostaglandine (PG) like compounds that are produced independently of the cyclooxygenase enzyme by free radical catalysed-peroxidation of arachidonic acid, and similar products are also formed during oxidation of EPA and DHA [9]. A substantial body of evidence indicates that measurement of F2 –IsoP levels (esterified in human tissues) provides a direct and reliable approach to assess oxidative damage in vivo compared with other methods (e.g.TBARS).
According to Barocas, et al. (2011) [42] the oxidative stress measured by urine F2-isoprostane level is strongly associated with prostate cancer.
Other bio-indicators
During the last decade, the development of immunochemical detection of HNE-histidine cytotoxic adducts (4-hydroxynonenal (4-HNE)), has opened more advanced methodological possibilities for qualitative and quantitative detection of lipid peroxidation in various human and animal tissues [43]. In addition, short chain hydrocarbon gases, e.g. ethane and pentane, are produced in vivo by thermal or ion catalysed decomposition of lipid hydroperoxides. Measurement of these exhaled gases by GC has therefore been largely applied to assess lipid peroxidation [44].
Biological antioxidants
The term biological antioxidants refer to compounds that protect biological systems against the potentially harmful effect of reactions that cause extensive oxidation [45]. They can act at several different stages in an oxidative sequence by:
- Removing oxygen or decreasing local O2 concentrations
- Removing catalytic metal ions or reactive oxygen species such as O·2- and H2O2
- Scavenging initiating radicals such as OH·, RO·, RO2·
Broadly, we distinguish between two types of biological antioxidants:
(a) Endogenous (intracellular) antioxidants: Oxygen metabolism occurs within cells where a variety of enzymes and proteins are acting specifically to remove oxygen intermediates. Such substances include catalase, selenium dependent glutathione peroxidase, copper and zinc-dependent superoxide dismutase, uric acid, and the transition metal-binding proteins, such as transferrin and caeruloplasmin [9]. These compounds are called endogenous antioxidants and offer protection at several different levels within the cells for example by preventing radical formation, repairing oxidative damage and increasing elimination of damaged molecules [38].
(b) Dietary antioxidants /focus of the current analysis: In addition to the endogenous antioxidants, nature has offered a wide range of nutritional compounds with strong antioxidant activities. Among these certain fat- or water-soluble vitamins can act as radical scavengers in model biological systems and in the human organism [46]. This paper focuses on selected compounds with vitamin or provitamin A activity given that tissues deficient in such nutrients may be prone to harmful peroxidation reactions and more specifically:
- Vitamin E: It comprises a category of eight monophenolic compounds (known as Tocopherols and tocotrienols) with strong reported antioxidant activities in food and biological systems, mainly acting as chain breaking antioxidants that inactivate free radicals via their hydrogen donating character [10]
- Vitamin C: A widespread vitamin in nature -in many fruits and vegetables- well known as antioxidant with multi-functional effects (incl. metal chelating properties) that can act synergistically with chain breaking antioxidants (e.g. tocopherols, flavonoids) resulting into synergistic effects [9]
- Provitamin A: (α, β-carotenes and β-cryptoxanthin): 40-carbon terpenoids widely available in nature with well-known scavenging activities against free radicals that are trapped in their conjugated structure. [5].
- Since the above-mentioned antioxidants are exogenous in nature, their levels can be manipulated by supplements and dietary modifications Natural occurrence, chemical structure and mechanism of antioxidant action of the above dietary antioxidants have been detailed by various researchers including Vance, et al. 2013 [47] and Kiokias, et al. 2009 [48]. Arvanitoyannis, et al. (2009) [49] reviewed the various available methods for the determination of chain-breaking antioxidant activity in food and biological systems. The total peroxyl radical trapping parameter (TRAP) and the oxygen radical absorbance capacity assay (ORAC) have served as the most commonly used methods of antioxidant capacity in food and biological systems during the last two decades [50,51]. Section 3 provides more details about the in vivo activity of antioxidant vitamins against LDL and DNA oxidative damage.
Human studies on the effect of natural antioxidant vitamins against LDL damage/atherosclerosis
(a) Effect of vitamins as individual compounds against LDL oxidation: A large number of studies have examined the effects of antioxidant vitamins either individually or in combination, on ex vivo LDL oxidation with most information being available for the effects of provitamins A, vitamins C and vitamin E [38].
Provitamin A carotenoids. Major non-provitamin A carotenoids (lycopene, lutein, and zeaxanthin) and provitamin A compounds carotenoids (Figure 2) have different biological activities and efficacy, depending on their food content, dietary intake, bioavailability, and bioconversion [52]. Epidemiologic studies have shown that diets rich in provitamin A-containing foods are associated with a decreased risk of conducting cardiovascular problems and other pathologic conditions [53]. The disease-preventing activity of β-carotene and other provitamin A carotenoids could be ascribed either to their conversion into retinoid or to their activity as intact molecules. The results of several human intervention studies, however, indicate that a high-dose supplementation with β-carotene, not only does not significantly decrease the risk for development for atherosclerosis, but could even be harmful to smokers or former asbestos workers [54]. Thus, it may be that β-carotene and other carotenoids (e.g. astaxanthin) promote health when supplemented at physiologic amounts in foods but could even present adverse prooxidant harmful activities when given in high doses and under highly oxidative conditions [44].
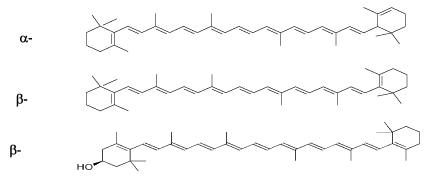
Figure 2. Structure of the provitamin A carotenoids (Kiokias 2002) [10].
Vitamin C. Ascorbic acid is a water-soluble antioxidant, and as such is expected to be removed from LDL during isolation. In an earlier study, Wen, et al. (1997) [55] did not find any significant effect of dietary supplementation with vitamin C on LDL oxidation in smoking volunteers. On the contrary other researchers have reported beneficial effects of dietary supplementation with 1000 mg vitamin C for 4 weeks [56] or 500 mg/d for 2 months [57] against LDL oxidative deterioration. In addition, McRae (2008) [58] noted that Vitamin C supplementation reduced serum LDL and triglycerides levels.
Vitamin E: Hodis, et al. (2012) [59] reported that α-tocopherol supplementation (400 IU/day) significantly raised plasma vitamin E levels (P< 0.0001) whereas reduced circulating oxidised-LDL (P= 0.03) and LDL oxidative susceptibility (P< 0.01). Studies that included vitamin E supplementation generally support a significant protection of LDL usually at doses higher than 400 IU/day [60]. As noted by Rizvi, et al. (2014) [61] Vitamin E is the major lipid-soluble component in the cell antioxidant defense system with numerous important roles within the body because of its antioxidant activity. However, it has also been suggested that when accompanying antioxidants such as ubiquinone and vitamin C are not available (to react and quench the vitamin E radicals) a prooxidant tocopherol effect could be induced [44]. Actually Niki (2010) [62] noted that recent human clinical trials with vitamin E have not yielded positive results against LDL oxidative deterioration on the contrary to in vitro experiments.
(b) Effect of vitamins' antioxidant mixtures against LDL oxidation:A growing body of human clinical investigation has focused into combination of various antioxidants in order to explore the potential of interactions because of varying modes and mechanisms of antioxidant action [9]. Interestingly, a few studies that were designed to supplement subjects with mixtures of antioxidant vitamins in generally observed an enhanced beneficial effect on oxidative stability of LDL, presumably resulting from a synergistic action between the vitamins [44,54]. Cocate, et al. (2015) [63] have recently conducted a cross-sectional study and observed that the total daily carotenoid intake of provitamin A carotenoids (β-cryptoxanthin, β and α-carotene lycopene) mixtured with xanthophylls (lutein plus zeaxanthin,) was inversely associated (p<0.05) with the plasma oxidised-LDL concentrations. Kiokias and Gordon (2003) [27] supplemented for 3 weeks 30 healthy volunteers with a carotenoid mixture (palm oil carotenes, lycopene, paprika, lutein, bixin in a total amount of 30 mg active carotenoid /day) and reported an increased resistance of LDL to oxidation, compared with placebo (monitored by CD at 233 nm).
Boushehri, et al. (2012) [64] examined the effect antioxidant vitamins on serum oxidized LDL levels in male subjects with cardiovascular disease risk factors. They reported that a diet enriched with a combination of vitamin C (500 mg), vitamin E (400 IU), ß-carotene (15 mg), was strongly associated with lower serum oxidized LDL levels. Similarly, in an earlier study [65] (Nyssonen, et al. 1994), a daily supplementation for 2 months with a mixture of vitamin E (200 mg) together with vitamin C (400 mg) and β-carotene (20 mg) decreased significantly the susceptibility of LDL to oxidative deterioration.
Human studies on the effects of antioxidant vitamins against DNA damage/carcinogenesis
(a) Effect of vitamins as individual compounds: Steady state estimates of cellular DNA oxidation, in general have provided support for a beneficial role of antioxidant vitamins in DNA protection [66].
Provitamin A compounds: Lorenzo, et al. (2009) [67] have investigated the biological properties of beta-cryptoxanthin, in cell culture model systems, using the comet assay to measure DNA damage. They reported that at low concentrations, close to those found in plasma, beta-cryptoxanthin does not itself cause damage, but rather protects transformed human cells from damage induced by H(2)O(2) or by visible light in the presence of a photosensitizer.
Astley, et al. (2004) [68] supplemented healthy male volunteers with lutein, beta-carotene or lycopene (natural isolate capsules, 15 mg/d, 4 weeks) and observed that both beta carotene and non-provitamin A carotenoids exerted an antioxidant protection by scavenging DNA-damaging free radicals and modulation of DNA repair mechanisms. On the other side, Collins and Azqueta and (2012) [34] stated that studying reports from the last 5 years, revealed a clear distinction between effects of pro-vitamin A carotenoids (carotenes and β-cryptoxanthin) and the effects of non-vitamin A carotenoids (lycopene, lutein, astaxanthin and zeaxanthin). Whereas the compounds of the latter group are almost invariably reported to protect against DNA damage, the provitamin A carotenoids show a more varied spectrum of effects, sometimes protecting and sometimes enhancing DNA damage.
Vitamin E: Makpol, et al. (2010) [69] observed that alpha-tocopherol protected against H(2)O(2)-induced DNA damage and this modulation was affected by donor age. Ragin, et al. (2010) [70] conducted a human trial showing that an intake of food rich in α-tocopherol could decrease levels of DNA oxidative adducts. Asgard (2014) [71] reported a significant decrease of catechol-induced (1 mM) general DNA damage in the presence of 20 μM of α-tocopherol. On the contrary, Barcelos, et al. (2011) [72] supported that dietary supplementation with α-tocopherol can even induce DNA oxidative stress.
Vitamin C: A protective effect of vitamin C supplementation in human with plasma levels>50μmol/l was observed by Sram, et al. (2012) [73] in terms of 8-oxodG levels. Similarly, in an earlier study, Kadirvel, et al. (2007) [74] reported that supplementation with ascorbic acid significantly prevents the arsenic-induced protein oxidation and DNA damage in rats. More recently, Kontek, et al. (2013) [75] noted that vitamin C (in a concentration range 10-100 μm) caused a clear protecting effect against DNA damaging. More specifically, they concluded that vitamin C modulates DNA damage induced by hydrogen peroxide in human colorectal adenocarcinoma cell lines (HT29), estimated by COMET assay in vitro (decrease ∼ 30%). Asgard (2014) [71] reported that high plasma levels of ascorbate reduced the levels of oxidative DNA damage (8- oxodG) in mononuclear white blood cells. Overall, Konopacka (2004) [76] highlighted that data concerning the influence of vitamin C on oxidative DNA damage are conflicting and some of the discrepancies can be explained by the different experimental methodologies employed.
(b) Effect of vitamins in antioxidant mixtures: In an earlier study, Sweetman, et al. 1997 [77] had examined the effect of antioxidant vitamin supplementation on DNA damage and repair in human lymphoblastoid cells. After 24-hour supplementation period with a mixture ascorbic acid + alpha-tocopherol, (60 microM in total) the level of endogenous DNA damage was significantly lower than in the nonsupplemented culture, as assessed by the comet assay. In addition, a human clinical trial [78] by Duthie, et al. (1997) reported that supplementation of smokers and non-smokers with an antioxidant mixture (vitamin C-100 mg +vitamin E-280 mg +and β-carotene-25 mg per day) significantly (p<0.002) reduced base damage in lymphocyte DNA. These findings agree with another human trial [79] where the researchers conducted a dietary intervention trial with 23 healthy subjects (after 2 weeks of washout) supplemented daily with 40 mg lycopene (weeks 3 and 4), 22.3 mg β-carotene and 15.7 mg α-carotene (weeks 5 and 6), and 11.3 mg lutein (weeks 7 and 8) resulting into significantly decreased DNA damage in lymphocytes. Kiokias & Gordon (2003) [27] conducted a double-blind, placebo-controlled cross over study with 30 healthy subjects. Following a dietary supplementation of 30 mg carotenoid mixture /day (a,b-carotene, lycopene, paprika, lutein, bixin, total amount) they reported a significant effect against production of urinary 8-OHdG estimated by use of ELISA test.
More recently, Cocate, et al. (2015) [63] conducted a cross-sectional study with the participation of 296 apparently healthy middle-aged men to assess the potential relationships of carotenoid intake with lipid and oxidative stress markers.
In conclusion, the total daily carotenoid intake based on five investigated carotenoid types (β-cryptoxanthin, lycopene, lutein plus zeaxanthin, β-carotene and α-carotene) was inversely associated with the production of urinary 8-OH-dG as oxidative stress biomarker (p<0.05). On the contrary, Asgard (2014) [72] reported that supplementation of 47 type-2 diabetes subjects for 12 weeks with 16 capsules/day (mixture of β-carotene and α-tocopherol) did not exert any inhibitory effect against DNA oxidative stress.
An overview on the human trial that investigated into antioxidant effects of vitamins combinations against LDL and/or DNA oxidative changes is given in (Table 2).
Table 2. Selection of human clinical trials designed to examine the effect of dietary supplementation with vitamins mixtures against LDL and/or DNA oxidative changes (current analysis).
Authors/studies
(listed in date order) |
Condition of Human
Clinical trials |
Protective (or not) effect of the vitamin mixture |
|
|
Effect against LDL oxidation |
Effect against DNA damage |
Duthie, et al. (1997) [78] |
Supplementation of smokers and non-smokers with an antioxidant mixture (vitamin C-100 mg +vitamin E-280 mg +and β-carotene-25 mg per day) |
---------- |
Significantly (p<0.002) reduced
base damage in lymphocyte DNA. |
Kiokias & Gordon (2003) [27] |
30 healthy volunteers were supplemented for 3 weeks with a mixture of 30 mg active carotenoid/day containing palm oil carotenes, lycopene, paprika, lutein, bixin). |
A reduction of ex vivo LDL oxidative modification (monitored
by conjugated dienes at 233 nm |
A reduction of in vivo oxidative
DNA damage by measuring
8-OHDG adduct (ELISA method) |
Astley, et al. (2004) [68] |
Healthy males were supplemented with 15 mg/d of lutein, β-carotene or lycopene (natural isolate capsules) for 4 weeks (3 independent clinical trials) |
---------- |
Carotenoids presented an antioxidant character by scavenging DNA-damaging free radicals
|
Boushehri, et al. (2012) [64] |
Male subjects followed a diet enriched with a combination of vitamin C (500 mg), vitamin E (400 IU), ß-carotene (15 mg), |
The antioxidant treatment resulted
into significantly lower
serum oxidized LDL levels. |
---------- |
Asgard (2014)
[72] |
47 type-2 diabetes subjects supplemented for 12 weeks with 16 capsules/day (mixture of β-carotene + α-tocopherol) |
---------- |
Dietary supplementation did not significantly reduce biomarkers of oxidative stress despite the substantial increased of plasma vitamin concentrations |
Cocate, et al. (2015)
[63]
|
296 healthy middle-aged subjects were supplemented with a carotenoid mixture (β-cryptoxanthin, lycopene, lutein plus zeaxanthin, β-carotene and α-carotene). |
The carotenoid supplementation resulted into reduced plasma oxidised-LDL concentrations. (p<0.05)
|
---------- |
The review of the earlier indicated studies on oxidative stress and effect of antioxidant vitamins has led to the following conclusions in the frame of the current analysis:
(a) Relatively low levels of LDL enrichment in provitamin A (<30 mg/day) can exert a better protective effect against oxidation of LDL ex vivo than higher doses of carotenoid supplements (60-100 mg of carotenoids/day) that fail to present any activity. An explanation for this is that a dietary intervention resulting in high carotenoid enrichments may have excessively loaded LDL particles with carotene autoxidation metabolites that can generate reactive oxygen species (leading thereby to increased LDL oxidative susceptibility rather than to any protection effect).
(b) A recent body of clinical research evidence has concluded that dietary combination of vitamins (e.g. vitamin E and vitamin C) can be more effective against oxidative damage of either LDL or DNA than the supplementation of each individual vitamin. Such an enhanced effect of vitamins mixtures may relate to the different mode of activities of the individual compounds thereby allowing a synergistic effect when combined in the diet. In particular for carotenoids, a better antioxidant effect against oxidative damage has been reported when provitamin A compounds (mainy hydrophobic α- and β-carotene) are supplemented together with preparations of more polar xanthopylls (e.g. lutein or paprika) in recent human clinical studies.
(c) The association between plasma oxidized LDL and DNA oxidative adducts with the eventual risk of developing a cardiovascular disease or cancer respectively are not completely elucidated yet. Further investigation is required in this filed to obtain more recent and valid clinical intervention data further to the existing epidemiological data. The development of optimal nutritional and future health strategies would certainly be facilitated by further investigation into the clinical effects of combined vitamins dietary supplementation against oxidative pathological conditions.
- Preiser J (2015) Oxidative stress. J Parent Enter Nutrit 36: 147-154.
- Lien P, Chuong PH (2008) Free radicals, antioxidants in disease and health. Int J Biomed Sci 4: 89–96. [Crossref]
- Kumar S (2014) The Importance of Antioxidant and their role in Pharmaceutical science. As J Res Chem Pharma Sci 1: 27-44.
- Scott TL, Rangaswamy S, Wicker CA, Izumi T (2014) Repair of Oxidative DNA Damage and Cancer: Recent Progress in DNA Base Excision Repair. Antioxid Redox Signal 20:708-726. [Crossref]
- Kiokias S, Oreopoulou V (2006) Antioxidant properties of natural carotenoid preparations against the AAPH-oxidation of food emulsions. Innovat Food Sci Emerg Technol 7: 132-139.
- Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev 4: 118–126. [Crossref]
- Gago-Dominguez M, Castelao JE (2008) Role of lipid peroxidation and oxidative stress in the association between thyroid diseases and breast cancer. Crit Rev Oncol Hematol 68: 107-114. [Crossref]
- Chao Y, Wei N, Mikko A, Matti PR, Manjula RC, et al. (2016) Source characterization of highly oxidized multifunctional compounds in a boreal forest environment using positive matrix factorization. Atmosph Chem Phys 16: 12715-12731.
- Kiokias S, Proestos C, OreopoulouV (2018) Effect of natural food antioxidants against LDL and DNA oxidative changes. Antioxidants 7: 133. [Crossref]
- Kiokias S (2002) In vitro and in vivo antioxidant activities of natural carotenoids. PhD thesis. The University of Reading.
- Krauss RM (2010) Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol 21: 305-311. [Crossref]
- Zhao X, Sun D, Xu RX, Guo YL, Zhu CG, et al. (2018) Low-density lipoprotein-associated variables and the severity of coronary artery disease: an untreated Chinese cohort study. Biomarkers 23: 647-653. [Crossref]
- Kratz M, Baars T, Guyenet S (2013) The relationship between high-fat dairy consumption and obesity, cardiovascular, and metabolic disease. Eur J Nutr 52: 1–24. [Crossref]
- Parthasaranthy S, Raghavamenon A, Raghavamenon S, Garelnabi OM, Santanam N (2010) Oxidized low-density lipoprotein. Methods Mol Biol 610: 403–417. [Crossref]
- Iqbal MP (2014) Trans fatty acids – A risk factor for cardiovascular disease. Pak J Med Sci 30: 194–197. [Crossref]
- Fang R, Zhang N, Wang C, Zhao X, Liu L, et al. (2011) Relations between plasma ox-LDL and carotid plaque among Chinese Han ethnic group. Neurol Res 33: 460-466. [Crossref]
- García-Gómez C, Bianchi M, de la Fuente D, Badimon L, Padró T, et al. (2014) Inflammation, lipid metabolism and cardiovascular risk in rheumatoid arthritis: A qualitative relationship? World J Orthop 5: 304-311. [Crossref]
- Tsimikas S, Kiechl S, Willeit J, Mayr M, Miller ER, et al. (2006) Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five year prospective results from the Bruneck study. J Am Coll Cardiol 47: 2219-2228. [Crossref]
- Valko M, Izakovic M, Mazur M, Rhodes JC, Telser J (2004) Oxidative damage and cancer incidence. Mol Cell Biochem 266: 37–56.
- Kaur V, Kumar M, Kumar A, Kaur K, Dhillon VS, et al. (2017) Pharmacotherapeutic potential of phytochemicals: Implications in cancer chemoprevention and future perspectives. Biomed Pharmacother 97: 564–586. [Crossref]
- Nandhakumar S, Parasuraman S, Shanmugam MM, Rao KR, Chand P, et al. (2011) Evaluation of DNA damage using single-cell gel electrophoresis (Comet Assay). J Pharmacol Pharmacother 2: 107–111. [Crossref]
- Watson J (2013) Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol 3: 120-144. [Crossref]
- Shaposhnikov S, Thomsen PD, Collins AR (2011) Combining fluorescent in situ hybridization with the comet assay for targeted examination of DNA damage and repair. Methods Mol Biol 682: 115–132. [Crossref]
- Singh N, Singh N, Singh SK, Singh AK, Bhargava V (2010) Status of LDL oxidation and antioxidant potential of LDL in type II diabetes. Biomed Res 21: 416-418.
- Slyskova J, Langie AS, Collins AR, Vodicka P (2014) Functional evaluation of DNA repair in human biopsies and their relation to other cellular biomarkers. Front Genet 5: 123. [Crossref]
- Kiokias S, Varzakas T (2014) Activity of flavonoids and beta-carotene during the auto-oxidative deterioration of model food oil-in water emulsions. Food Chem 150: 280-286. [Crossref]
- Kiokias S, Gordon MH (2003) Dietary supplementation with a natural carotenoid mixture decreases oxidative stress. Eur J Clin Nutr 57: 1135-1140. [Crossref]
- Amarowicz R, Pegg RB (2017) The potential protective effects of phenolic compounds against low-density lipoprotein oxidation. Curr Pharm Des 23: 2754-2766. [Crossref]
- Winklhofer-Rooba B, Faustmanna G, Roob JM (2017) Low-density lipoprotein oxidation biomarkers in human health and disease and effects of bioactive compounds. Free Radic Biol Med 111: 38–86. [Crossref]
- Pizzimenti S, Ciamporcero E, Daga, M, Pettazoni, E, Arcaro, A et al. (2013) Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front Physiol 4: 242-247. [Crossref]
- Damas J, Carneiro J, Gonçalves J, Stewart JB, Samuels DC, et al. (2012) Mitochondrial DNA deletions are associated with non-B DNA conformations. Nucleic Acids Res 40: 7606-7621. [Crossref]
- Valavanidis A, Vlachogianni T, Fiotakis C (2009) 8-hydroxy-2' -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Enviro Sci Health Part C Environmental Carcinog 27: 120-139.
- Toyokuni S (1999) Reactive oxygen species-induced molecular damage and its application in pathology. Pathol Int 49: 91-102. [Crossref]
- Collins AR, Azqueta A (2012) DNA repair as a biomarker in human biomonitoring studies: further applications of the comet assay. Mutat Res 736: 122–129. [Crossref]
- Hofer T, Karlsson HL, Möller L (2006) DNA oxidative damage and strand breaks in young healthy individuals: a gender difference and the role of life style factors. Free Radic Res 40: 707-714. [Crossref]
- Ersson C, Möller L (2011) The effects on DNA migration by altering parameters in the comet assay protocol, including agarose density, electrophoresis conditions as well as durations of enzyme and alkaline treatments. Mutagenesis 26: 689-695. [Crossref]
- Kiokias S, Dimakou C, Tsaprouni I, Oreopoulou V (2006) Effect of compositional factors against the thermal oxidation of novel food emulsions. Food Biophysics 1: 115-23.
- Kiokias S, Varzakas T, Arvanitoyannis I, Athanasios L (2009) Lipid oxidation and its control. In Advances in Food Biochemistry, chapter-12, CRC Press, New York, 384-403
- Dimakou C, Kiokias S, Tsaprouni I, Oreopoulou V (2007) Effect of processing and storage parameters on oxidative deterioration of oil-in-water emulsions. Food Biophysics 2: 38-45.
- TJN Okonkwo, CJO Okonkwo (2009) Antioxidant Properties of Diospyros Preussi (Ebenaceae Gurke) Seed Oil Tropical. J Pharmaceut Res 8: 6.
- Pandey MC, Harilal PT, Radhakrishna K (2014) Effect of processing conditions on physico-chemical and textural properties of shami kebab. Int Food Res J 21: 223–228.
- Barocas DA, Motley S, Cookson MS, Chang SS, Penson DF, et al. (2011) Oxidative stress measured by urine F2-isoprostane level is associated with prostate cancer. J Urol 185: 2102-2107. [Crossref]
- Weber D, Milkovic L, Bennett SJ, Griffiths HR, Zarkovic N, et al. (2013) Measurement of HNE-protein adducts in human plasma and serum by ELISA—Comparison of two primary antibodies. Redox Biol 1: 226–233. [Crossref]
- Kiokias S, Gordon M (2004) Properties of carotenoids in vitro and in vivo. Food Rev Int 20: 99-121.
- Rizzo AM, Berselli P, Zava S, Montorfano G, Negroni M, et al. (2010) Endogenous antioxidants and radical scavengers. Adv Exp Med Biol 698: 52-67. [Crossref]
- Takashima M, Horie M, Shichirini M, Hagihara Y, Yoshida Y, et al. (2012) Assessment of antioxidant capacity for scavenging free radicals in vitro: A rational basis and practical application. Free Radic Biol Med 52: 1242–1252. [Crossref]
- Vance TM, Su J, Fontham TE, Koo SI, Chun OK (2013) Dietary Antioxidants and Prostate Cancer: A Review. Nutr Cancer 65: 793-801. [Crossref]
- Roginsky V, Lissi AE (2005) Review of methods to determine chain-breaking antioxidant activity in food. Food Chem 92: 235-254.
- Arvanitoyannis I, Varzakas T, Kiokias S, Labropoulos A (2009) Lipids, Fats & Oils. In Advances in Food Biochemistry, chapter-5, CRC Press, New York, 132-190.
- Ou B, Hampsch-Woodill M, Prior R (2001) Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem 49: 4619–4626. [Crossref]
- Pellegrini N, Serafini M, Colombi B, Del Rio D, Salvatore S, et al. (2003) Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J Nutr 133: 2812-2819. [Crossref]
- Toti E, Chen CY, Palmery M, Valencia VD, Peluso I (2018) Non-Provitamin A and Provitamin A Carotenoids as Immunomodulators: Recommended Dietary Allowance, Therapeutic Index, or Personalized Nutrition? Oxid Med Cell Longev 4637861. [Crossref]
- Tang G (2010) Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am J Clin Nutr 91: 1468S-1473S. [Crossref]
- Kiokias S, Varzakas T, Oreopoulou V (2008) In vitro activity of vitamins, flavonoids, and natural phenolic antioxidants against the oxidative deterioration of oil-based systems. Crit Rev Food Sci Nutr 48: 78–93. [Crossref]
- Wen Y, Cooke T, Feely J (1997) The effect of pharmacological supplementation with Vit-C on LDL oxidation. Br J Clin Pharmacol 44: 94-97. [Crossref]
- Fuller CJ, Grundy SM, Norkus EP, Jialal I (1996) Effect of acsorbate supplementation on LDL oxidation in smokers. Atherosclerosis 119: 139-150. [Crossref]
- Harats D, Chevion S, Nahir M, Norman Y, Sagee O, et al. (1998) Citrus fruit supplementation reduces lipoprotein oxidation in young men ingesting a diet rich in saturated fat, presumptive evidence for interaction between Vit-C and Vit-E in vivo. Am J Clin Nutr 67: 240-245. [Crossref]
- McRae MP (2008)Vitamin C supplementation lowers serum low-density lipoprotein cholesterol and triglycerides: a meta-analysis of 13 randomized controlled trials. J Chiropr Med 7: 48–58. [Crossref]
- Hodis HN, Mack WJ, LaBree L, Mahrer PR, Sevabian A, et al. (2002) Alpha-tocopherol supplementation in healthy individuals reduces low-density oxidation but not atherosclerosis. Circulation 106: 1453–1459. [Crossref]
- Jialal I, Fuller CJ, Huet BA (1995) The effect of a-tocopherol supplementation on LDL oxidation- a dose response study. Arterioscler Thromb Vasc Biol 15: 190-198. [Crossref]
- Rizvi S, Raza TS, Ahmed, F, Ahmad, A, Abbas S, et al. (2014) The Role of Vitamin E in Human Health and Some Diseases. Sultan Qaboos Univ Med J 14: e157–e165. [Crossref]
- Niki E (2010) Assessment of antioxidant capacity in vitro and in vivo. Free Radic Biol Med 49: 503–515. [Crossref]
- Cocate PG, Natali AJ, Alfenas RG, de Oliveira A, dos Santos EC, et al. (2015) Carotenoid consumption is related to lower lipid oxidation and DNA damage in middle-aged men. Br J Nutr 114: 257-264. [Crossref]
- Najafpour Boushehri S1, Yusof RM, Nasir Mohammad Taib M, Mirzaei K, Yazdekhasti N, et al. (2012) Effect of Vitamin Supplementation on Serum Oxidized Low-Density Lipoprotein Levels in Male Subjects with Cardiovascular Disease Risk Factors. Iran J Basic Med Sci 15: 958–964. [Crossref]
- Nyssonen K (1994) Increase in oxidation resistance of atherogenesis in serul lipoproteins following antioxidant supplementation. A randomized-double-blind-placebo-clinical-trial. Eu J Clin Nut 18: 633-642.
- Nderitu AM, Dykes L, Awika MJ, Minnaar A, Duodu GK (2013) Phenolic composition and inhibitory effect against oxidative DNA damage of cooked cowpeas as affected by simulated in vitro gastrointestinal digestion. Food Chem 141: 1763-1771. [Crossref]
- Lorenzo Y, Azqueta A, Luna L, Bonilla F,Domínguez G, et al. (2009) The carotenoid beta-cryptoxanthin stimulates the repair of DNA oxidation damage in addition to acting as an antioxidant in human cells. Carcinogenesis 30: 308-314. [Crossref]
- Astley SB, Hughes DA, Wright AJ, Elliott RM, Southon S (2004) DNA damage and susceptibility to oxidative damage in lymphocytes: effects of carotenoids in vitro and in vivo. Br J Nutr 91: 53-61. [Crossref]
- Makpol S, Zainuddin A, Rahim NA, Yusof YA, Ngah WZ (2010) Alpha-tocopherol modulates hydrogen peroxide-induced DNA damage and telomere shortening of human skin fibroblasts derived from differently aged individuals. Plant Med 76: 869-875. [Crossref]
- Ragin C, Minor A, Farmer P, Garte S, Gonzales C, et al. (2010) Pooled analysis of studies on DNA adducts and dietary vitamins. Mut Res 705: 77-82. [Crossref]
- Asgard R (2014) Effects of antioxidants and prooxidants on oxidative stress and DNA damage using the comet assay. Digital Comprehensive Summaries of Dissertations. Faculty of Pharmacy, University of Uppsala, 22-30.
- Barcelos GR, Grotto D, Serpeloni JM, Angeli JP, Rocha BA, et al. (2011) Protective properties of quercetin against DNA damage and oxidative stress induced by methylmercury in rats. Arch Toxicol 85: 1151-1157. [Crossref]
- Sram RJ, Binkova B, Rossner P Jr (2012) Vitamin C for DNA damage prevention. Mutat Res 733: 39-49. [Crossref]
- Kardivel R, Sundaram K, Mani S, Samuel S, Elango N, et al. (2007) Supplementation of ascorbic acid and alpha-tocopherol prevents arsenic-induced protein oxidation and DNA damage induced by arsenic in rats. Hum Exp Toxicol 26: 939-946. [Crossref]
- Kontek R, Kontek B, Grzegorczyk K (2013) Vitamin C modulates DNA damage induced by hydrogen peroxide in human colorectal adenocarcinoma cell lines (HT29) estimated by comet assay in vitro. Arch Med Sci 9: 1006–1012. [Crossref]
- Konopacka M (2004) Role of vitamin C in oxidative DNA damage. Postepy Hig Med Dosw 58: 343-348. [Crossref]
- Sweetman SF, Strain JJ, McKelvey-Martin VJ (1997) Effect of antioxidant supplementation on DNA damage and repair in human lymphoblastoid cells. Nutr Cancer 27: 122–130. [Crossref]
- Duthie G (1999) Determination of activity of antioxidants in human subjects. Proc Nutr Soc 58: 1015-1024. [Crossref]
- Pool-Zobel BL, Bub A, Muller H, Wollowski I, Reckemmer G (1997) Consumption of vegetables reduces genetic damage in humans: first results of a human intervention trial with carotenoid rich foods. Carcinogenesis 18: 1847-1850. [Crossref]


