Abstract
Clovamide is defined by acid-amide bonds between caffeic acid and 3, 4-dihydroxyphenylalanine (dopa), whereas caffeoyl tyrosine is defined by acid-amide bonds between caffeic acid and 4-hydroxyphenylalnine. We compared the protective effects of both compounds against a carbon tetrachloride (CCl4)-induced liver injury, measuring serum alanine amino transferase (ALT) activity in mice. We observed that clovamide, with its dopa moiety (orthodiphenol structure) and with higher DPPH radical scavenging activity than caffeoyl tyrosine had stronger lowering effect of ALT activity than caffeoyl tyrosine, which had a monophenol structural amino acid moiety.
The presence of glucuronide, methyl esters and sulfates in both of clovamide and caffeoyl tyrosine in the serum of mice administered clovamide and caffeoyl tyrosine, indicated that these compounds could be modified at the same hydroxyl positions of these compound, and metabolized in a similar route.
Keywords
Clovamide, Caffeoyl tyrosine, Liver injury amelioration, Metabolites, Carbon tetrachloride, Mouse injury model
Introduction
Acute and chronic liver injuries, which can result from exposure to oxidative stress and reactive oxygen species, are serious global issues. Numerous medicinal plant formulations have been generated to protect the liver from these conditions. Based on this, radical scavenging activities of formulation constituents have been functionally associated with their ability to reduce such liver injuries. Thus, compounds with potentially therapeutic activities have been identified and characterized, e.g., epi-gallocatechin gallate from green tea is a well-known radical scavenger, which ameliorates liver injury induced by carbon tetrachloride (CCl4) [1,2]. Similarly, flavonoids such as quercetin and isorhamnetin and their vegetable derivatives, and also chlorogenic acid and associated derivatives, have been shown to exert strong radical scavenging activities toward liver injury induced by CCl4 in mice [3-7]. However, the radical scavenging and the hepato-protective effects of clovamide (caffeoyl dopa), which is distinct in chemical structure to chlorogenic acid, have not yet been characterized in mice. Clovamide (PubChem CID: 6443790) is formed by acid-amide bonding between caffeic acid and dopa (3, 4-dihyroxyphenylalanine), whereas chlorogenic acid is formed by ester bonding between caffeic acid and quinic acid. Clovamide is found in cacao (especially, in the peel) and coffee beans [8] and is consumed in daily meal. Thus, it is important to ascertain if clovamide exerts hepato-protective effects using a mouse model, and to assess how the compound is metabolized. Additionally, although the anti-oxidative and neuro-protective activities of clovamide have been similarly reported in cell lines [9-12], the protective effects of clovamide and caffeoyl tyrosine (PubChem CID: 14352555) and their metabolites have not been adequately assessed against CCl4-induced liver injury in mice [8,13].
Caffeoyl tyrosine is also found in coffee beans and cocoa seeds [14] and is composed of caffeic acid and tyrosine via acid-amide bonding. Therefore, a comparative study of the hepato-protective effects of both caffeoyl tyrosine and clovamide is warranted, as both have the same caffeic acid moiety, but different binding moieties, i.e., dopa for clovamide and tyrosine for caffeoyl tyrosine.
Thus, hepato-protective activities were compared between these compounds, and the metabolites of both were investigated in mouse serum to find out if the metabolism of these compound in vivo is the same.
Materials and methods
The synthesis of clovamide and caffeoyl tyrosine
Clovamide was synthesized from caffeic acid and L-3, 4-dihydrophenylalanine methyl ester hydrochloride (Sigma-Aldrich, Inc., USA) in the presence of dicylohexylcarbodiimide (Sigma-Aldrich) and dry pyridine, according to the method of Tebayashi et al [15]. The synthesized crude clovamide was fractionated by cellulose (ADVANTEC, Japan) column chromatography, using 2% acetic acid as an eluent. The clovamide fractions was added to a DIAION HP-20 (Mitsubishi Chem. Co., Japan) column, followed by elution in methanol (MeOH), after washing the column in distilled water. The clovamide rich-fraction was further purified on a LH-20 column, with MeOH as an eluent. Further purification was conducted by preparative high performance liquid chromatography on a Develosil C-30 UG-5 (20 mm internal diameter (i.d.)×250 mm) column using a solvent system composed of 5% acetonitrile (MeCN)/1% acetic acid (solvent A) and 40% MeCN (solvent B). A linear gradient of solvent B in solvent A was used for the development of the solvent to isolate clovamide as a single peak on an analytical column (Develosil C-30 UG-5, 4.6 mm i.d. ×250 mm).
Caffeoyl tyrosine was synthesized from caffeic acid and tyrosine methyl ester hydrochloride (Sigma-Aldrich) and purified using the same method as clovamide.
Measurement of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity
The DPPH radical scavenging activity of clovamide and caffeoyl tyrosine was determined according to the methods of Suda [16] and Sugawara et al. [16,17], and expressed as the Trolox equivalent (mole/mole sample).
Animal studies
Animal care
These studies were approved by the Animal Research Committee in the Faculty of Agriculture, Yamagata University, Japan (IACUC number: 20-131). Mice were cared for according to the institutional guidelines of the Faculty of Agriculture, Yamagata University, and to guideline in the Helsinki Declaration to animal experiments.
Animals and sample administration
Male ddY mice (eight weeks old; mean body weight; 32 g; 36 mice) were purchased from Japan SLC. Inc (Hamamatsu, Shizuoke, Japan). Mice were individually housed in stainless-steel cages, with a 12 h light-12 h dark cycle at 22˚C ± 2˚C, and 60% humidity. A commercial diet (F-2 from Funabashi Farms, Funabashi, Japan) and water were provided ad libitum. After acclimatization for three days, mice were divided into six groups based on body weight and given a basal diet (AIN-93) [18] for two days. This diet comprised 20% casein, 62.95% α-corn starch: sucrose (2:1), 7% corn oil, 3.5% mineral mixture (AIN-93G-MX, Oriental Yeast Co., Tokyo, Japan), 1% vitamin mixture (AIN-93-VX, Oriental Co.), 0.30% L-cystine, and 0.25% choline bitartrate. The six groups were control (n=6), CCl4 (n=6), CCl₄+clovamide (n=6), CCl4+caffeic acid (n=6), CCl4+caffeoyl tyrosine (n=6), and CCl4+DOPA (n=6) groups. Groups of CCl4+caffeic acid and CCl4+DOPA were prepared to determine if the effects of clovamide are due to both moieties of caffeic acid and DOPA.
All mice were deprived of their basal diet for 8 h before the next treatments. Mice of the CCl4, CCl4+clovamide, CCl4+caffeic acid, CCl4+caffeoyl tyrosine, and CCl4+DOPA groups were administered, 0.12 mL of 0.5% carboxymethyl cellulose (CMC) solution (aqueous), 0.12 mL of 0.5% CMC containing 30mg clovamide, 0.12 ml 0.5%CMC solution containing 13.6 mg caffeic acid, 0.12 mL of 0.5% CMC solution containing 30 mg caffeoyl tyrosine and 0.12 mL of 0.5% CMC solution containing 16.4 mg DOPA, respectively, 30 min before intraperitoneal injection of 60 µL of CCl4 solution ( a 1:1 volume ratio of olive oil and CCl4). For the control group, mice were orally administered 0.12 mL of 0.5% CMC solution 30 min before 30µL of olive oil. Clovamide, caffeoyl tyrosine, caffeic acid, and DOPA were in equal molar quantities.
Blood was collected via heart puncture using nembutal (Dai Nippon Pharmaceutical Co., Ltd, Japan) anesthesia (intraperitoneal injection of 3.3 mL/kg/animal) at 22 h after i.p. injection of either CCl4 solution (for CCl4 and CCl4+sample groups) or olive oil (for the control group).
Serum preparation and alanine aminotransferase (ALT) measurements
Serum was prepared by centrifugation (1000 × g for 15 min), from which alanine aminotransferase (ALT) activities were measured according to the literature [17,19]. One unit of enzyme activity was defined as the amount of enzyme which transformed 1 µmol substrate per min per litre of serum (µmol/min/L of serum at 25˚C). Statistically significant differences were determined by Student’s t-tests, and a P < 0.05 was considered statistically significant.
Plasma metabolite preparation and measurements
To determine clovamide metabolites, approximately 0.2 mL serum from the clovamide group was mixed with 1.0 mL 0.067 M phosphate buffer (pH 5.0). The mixture was added to a DIAION HP-20 column (1 cm i.d. × 4 cm) equilibrated in distilled water, and then washed with distilled water. Metabolites were successively eluted with 50% and 100% MeOH. Both eluates were combined, evaporated, and re-dissolved in 2 mL MeOH. This was mixed with distilled water and chloroform (CHCl3) at a 1:2:1 ratio (MeOH:H2O:CHCl3, v/v/v). The upper layer was then evaporated and re-dissolved in a small MeOH volume before ultra-performance liquid chromatography (UPLC)-mass spectrometry (MS) analysis. Compounds were analyzed by high-resolution electro spray ionization ToF-mass spectroscopy (HRESI-Tof-MS) (Xevo Q-Tof MS, Waters, Manchester, UK) in the negative mode, using a BEH C-18 column (0.5 × 50 mm, 1.7 μm; Waters, Ireland). The Developing solvent comprised 0.1% HCOOH in water (solvent A) and 0.1% HCOOH in MeCN (solvent B). Developing solvent was eluted at a flow rate of 0.3 mL/min with gradient elution (0 min: 90% A, 6 min: 60% A, 7 min: 40% A, 8 min: 10% A, 9–13 min: 90% A). Cone and desolvation gas flows were 50 L/h and 800 L/h, respectively. The capillary voltage was 2.5 kV, the source temperature was 120°C, and the desolvation temperature was 400°C.
For caffeoyl tyrosine metabolite determination in mice, male C57BL mice (ten weeks old) were orally administered caffeoyl tyrosine (10 mg/0.5 ml 0.1% CMC) after over-night diet deprivation. Blood was collected from the heart 30 min after oral administration, and serum prepared by centrifuging bloods at 1000 × g for 15 min at 4°C, and mixed with 0.067 M phosphate buffer (pH 5.0) in a 1:2 ratio. This was followed by the same procedure as mice administered clovamide.
Results and discussion
The inhibitory activities of clovamide and caffeoyl tyrosine against CCl4-induced liver injury
Serum ALT activities in study mice are shown (Figure 1). When ALT activity was compared between the CCl4 group and the other groups (clovamide, caffeoyl tyrosine, caffeic acid, and DOPA groups), only the clovamide group showed significantly lower ALT levels (p<0.05). We observed no significant differences between the CCl4 group and caffeoyl tyrosine, caffeic acid, and DOPA groups, although ALT activity exhibited a reduced tendency in these groups. The strong suppression of ALT activity in the clovamide group when compared with the caffeoyl tyrosine group potentially indicated that the clovamide dopa moiety was more effective than the tyrosine moiety of caffeoyl tyrosine in suppressing increased ALT activity. As the DPPH radical scavenging activity of clovamide was stronger than caffeoyl tyrosine (Trolox equivalent in each compound (mole/mole of sample): clovamide = 0.81 and caffeoyl tyrosine = 0.08), this difference could be interpreted as the higher amelioration of livery injury by clovamide.
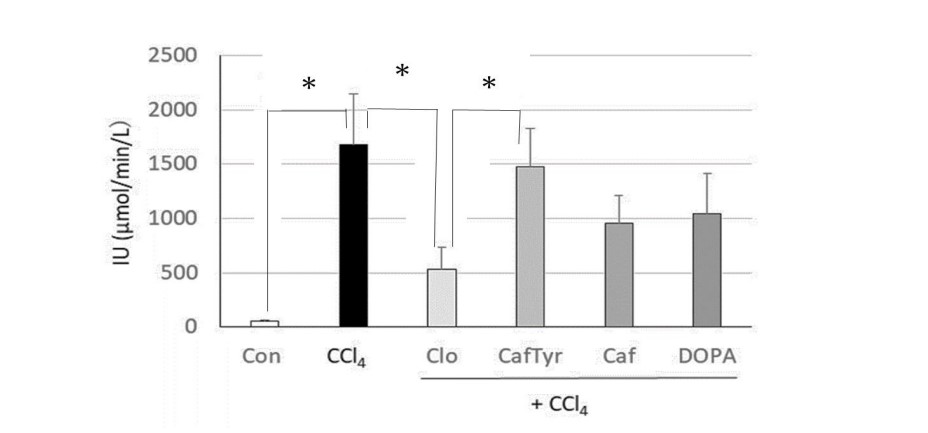
Figure 1: Effects of clovamide and its related compounds on serum ALT levels in a mouse liver injury model, induced by carbon tetrachloride (CCl4).
Con: Control group without oral administration of sample and intraperitoneal injection of CCl4. CCl4: Groups without oral administration of sample but intraperitoneal injection of CCl4. Samples (Clo, Caf Tyr, Caf and DOPA) were orally administered 30 min before intraperitoneal injection of CCl4 (Clo, Caf Tyr, Caf and DOPA groups, respectively) . Six mice were used in each group. * indicate significant differences between groups using Student's t-test (p < 0.05).
The identification of clovamide metabolites and associated metabolism
UPLC chromatogram measured at 330nm, and UV spectra of each peak on the chromatogram, in the serum of mice administered clovamide are shown in Figure 2A, and Figure 2B, respectively. As indicated in Figure 2B, peaks 1, 2, 4, 5, 8, 9, and 11 exhibited absorption maxima or shoulders at approximately 310–320 nm, showing these peaks were cinnamic acid-related compounds. Measured accurate mass of the pseudo-molecular ions ([M-H]-) and/or ([M-H]- +Na) of peaks 1, 2, 4, 5, 8, 9, and 11 (Table 1) showed good agreement with those calculated exact mass, indicating that each peak might depend the following compounds: peak 1, Clovamide-3’ or 4’-O-glucuronide; peak 2, Clovamide-3’ or 4’-O-glucuronide; peak 4, 3 or 4-O-Methyl-clovamide-3’ or 4’-O-glucuronide; peak 5, 3 or 4-O-, 3’ or 4’-O-Dimethyl-clovamide-3’ or 4’-O-glucuronide; peak 8, 3 or 4-O-Methyl-clovamide-3’ or 4’-O-sulfonate; peak 9, 3 or 4-O-, 3’ or 4’-O-Dimethyl-clovamide; and peak 11, 3 or 4-O-, 3’ or 4’-O-Dimethyl clovamide (Figure 3).
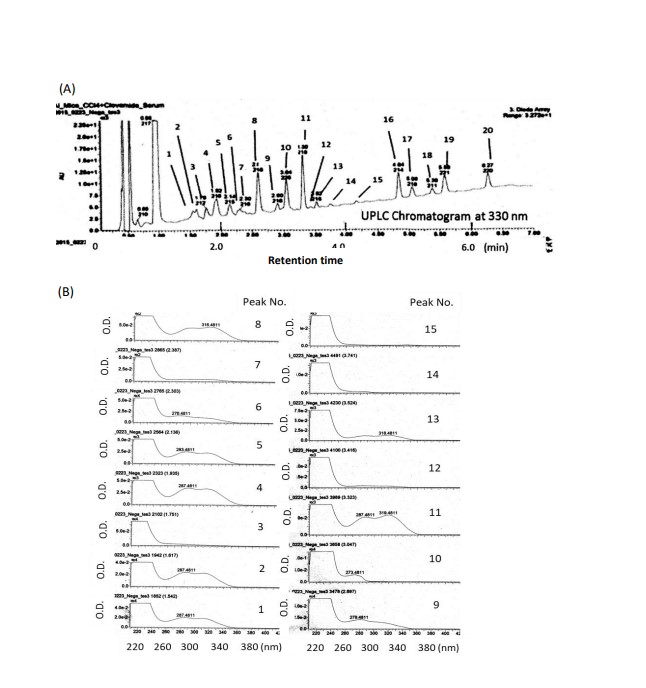
Figure 2: Ultra performance liquid chromatography (UPLC) (A) and UV specra of each peak No. (B) in serum of mice administered clovamide 30 min before intraperitoneal injection of carbon tetrachloride (CCl4), followed by blood collection 22 h later.
UPLC conditions: column, BEH C-18 (i.d. 2.1 × 5o mm, 1.7μm, Waters, Ireland) ; eluent, 0.1% HOOH (eluent A), 0.1%HCOOH in acetonitrile (eluent B); flow rate, 0.3mL/min; column temperature, 30 ℃ ; 0~100% B for 45 min with linear gradient; detection, 330 nm. Peaks 1, 2, 4, 5, 8, 9, and 11 had absorption maxa around 280-320 nm.
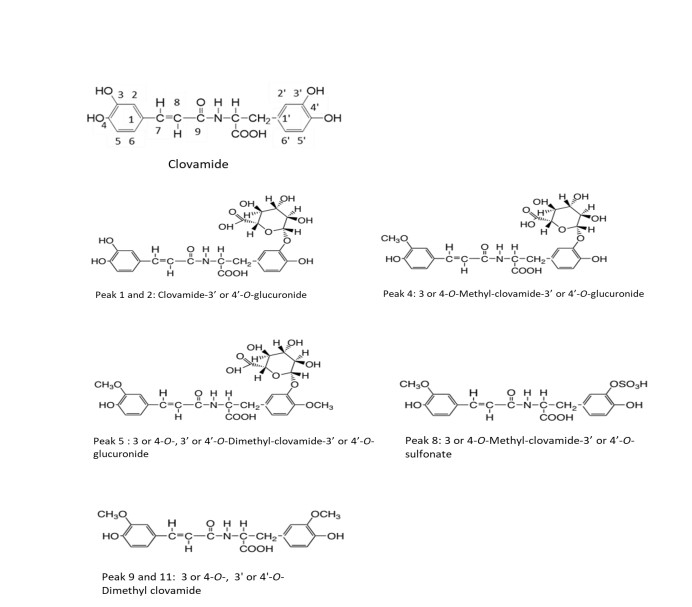
Figure 3: Metabolites in the serum of mice administered clovamide orally, 30 min before intraperitoneral injection of carbon tetrachloride and collected blood 22 h later.
From accurate mass numbers and elemental formulas (Table 1), the UV spectra of each peak, and previous reports showing that phenolic compounds administered to rats and mice could be metabolized to methylated compounds, glucuronides, and sulfates [20-25], the chemical structures of peaks 1, 2, 4, 5, 8, 9, and 11 were confirmed as shown in Figure 3.
These compounds in serum indicated that clovamide with acid-amide bonding is also glucuronidized, methylated and/or sulfated in gastro-intestinal tract and/or liver like rosmarinic acid with ester bonding between caffeic acid and 3,4-dihydroxy-benzenepropionic acid [26].
As methylation and/or glucuronidation of (-)-catechin at the 3’ or 4’ position of the B-ring are considered to decreases radical scavenging activities, the activity of identified metabolites, containing less hydroxyl groups than the original compound (clovamide), suggests they may be weaker than clovamide to reduce live injury. Previous reports suggesting that quercetin, curcumin, rosmarinic acid, and chlorogenic acid, having strong radical scavenging activities, may be glucuronidized and sulfated in vivo [20-25,27], potentially supported the notion that clovamide could be similarly metabolized and excreted.
The identification of caffeoyl tyrosine metabolites and associated metabolism
MS spectrum of ion corresponding to each ion peak observed at Rt (retention time) 1.958, 2.060, 2.475, and 2.910 min on the total ion chromatogram (TIC), in the serum of mice administered with caffeoyl tyrosine by single administration, and the chemical structure proposed from the MS spectrum are shown in Figure 4A–4D.

Figure 4: Mass Spectrometry (MS) spectra and proposed chemical structures of compounds with retention times at 1.958 min (compound A) (A), 2.060 min (compound B) (B), 2.475 min (compound C) (C), and 2.910 min (compound D) (D) on total ion chromatogram, in the serum of study mice.
The blood collected by heart puncture from mice 30 min after oral administration of caffeoyl tyrosine was centrifuged at 3000 rpm for 10 min, and the obtained serum, that was mixed with pH 6.0 buffer, was poured on to the Diaion column (1 i.d. x 3cm), then eluted with 50 % MeOH after washing the column with distilled water. The eluate (polyphenol fraction) was analyzed by UPLC-Xevo QTof MS equipment.
Compound A, (Figure 4A), showed pseudo molecular ion at m/z 532.1498 ([M-H]-) (532.1455, Calcd. for C25H26NO12) and its Na additive at m/z 554.1305 (554.12274, Calcd. for C25H25NO12Na,) in the MS spectrum of ion of ion peak extracted at Rt-1.958 min, and further, fragmentation ion observed at m/z 356.1154 (depend on methylated caffeoyl tyrosine moiety), indicating that compound A had the chemical structure; 3 or 4-O-methyl-caffeoyl tyrosine-4’-O-glucuronide.
Compound B, (Figure 4B), showed pseudo molecular ion at m/z 518.1316 (518.1299, Calcd. for C24H24NO12) and its Na additive at m/z 540.1128 (540.1118, Calcd. for C24H23NO12Na) in the MS spectrum of ion of ion peak extracted at Rt- 2.060 min on the TIC, and further, fragmentation ions at m/z 342.1023 (342.0978, Calcd. for C18H16NO6, depend on caffeoyl tyrosine moiety), indicating the chemical structure of compound B was caffeoyl tyrosine-4’-O-glucuronide.
Compound C, (Figure 4C), showed pseudo molecular ion at m/z 422.0545 (422.0546, Calcd. for C18H16NO9S) in the MS spectrum of ion of ion peak extracted at Rt-2.475 min on the TIC, and further, fragmentation ion at m/z 342.1013 (342.0978, Calcd. for C18H16NO6, depend on caffeoyl tyrosine moiety), indicating the chemical structure of compound C was caffeoyl tyrosine-4’-O-sulfate.
Compound D, (Figure 4D), showed the pseudo molecular ion at m/z 356.1154 (356.1134, Calcd. for C19H18NO6), and its Na additive at m/z 378.0954 (378.0954, Calcd. for C19H17NO6Na) in the MS spectrum of ion of ion peak extracted at Rt-2.910 min on the TIC, indicating that compound D was 3 or 4 or 4'-O-Methyl caffeoyl tyrosine.
Considering the molecular weights of fragmentation ions of compound A, it was hypothesized that compound A was glucuronidized at the 4’ position of caffeoyl tyrosine, and that methylation occurred at the 3 or 4 position of caffeoyl tyrosine. Measured accurate masses of pseudo molecular ion and its fragmentation ion in the Compound B supported that caffeoyl tyrosine could be glucuronidized at the 4’ position of caffeoyl tyrosine. Measured accurate masses of pseudo molecular ion and its fragmentation ion in the compound C indicated that sulfonation occurred at 4’ position of caffeoyl tyrosine. In compound D, the position of methylation is shown tentatively at 3’ position because that methylation also could occur at, 4 and 4’ positions of caffeoyl tyrosine.
The presence of these compounds in serum indicated that cinnamic acid derivatives composed of caffeic acid and amino acids, via acid-amide bonding, could be glucuronidized or sufonated on DOPA or tyrosine moieties, which are more hydrophilic than caffeic acid, in vivo. Similarly, methylation could occur on the caffeic acid moiety which is more hydrophobic than DOPA and tyrosine moieties, however more precise studies are required to confirm these observations. Although the presence of glucuronide and sulfate in the urine of healthy volunteers administered caffeoyl tyrosine could not be clearly confirmed [28], our data might suggest that caffeoyl tyrosine could be metabolized in vivo while undergoing glucuronidation and sulfation.
Conclusion
Clovamide, which showed higher DPPH radical scavenging activity than caffeoyl tyrosine was more effective in alleviating carbon tetrachloride-induced liver injury in mice. This result also showed that the dopa moiety with the ortho-dihydroxy group was more effective in alleviating liver injury than the tyrosine moiety with the monohydroxy group.
Methylated clovamide and its glucuronide and sulfate were found in serum of mice administered with clovamide. Methylated caffeoyl tyrosine, and its glucuronide and sulfate were found in the serum of mice administered with caffeoyl tyrosine. These findings indicated that both clovamide and caffeoyl tyrosine could be absorbed from the gastro-intestinal tract and metabolized undergoing methylation, glucuronidation, and sulfation, although it is necessary to examine if the identified metabolites are also effective for amelioration of liver injury (Figure 5).
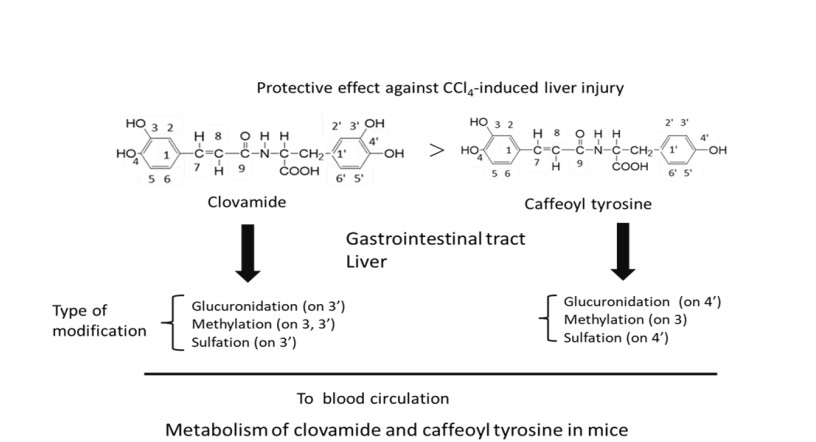
Figure 5: Proposed metabolism of clovamide and caffeoyl tyrosine in mice.
Declarations
Conflict of interest
The author declare no conflict of interest.
Authors’ contribution
Kiharu Igarashi conceived, and designed the experiments, and performed the experiments, data processing, and manuscript preparation.
Acknowledgments
The author would like to express my sincere gratitude to Ai Kaneko and Kayoko Sudo for their help with the experiment and data processing.
Funding
This study was partly supported by Grant-in Aid for Scientific Research (No.16658054, to K.I.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and partly by The Japan Food Chemical Research Foundation.
References
- Igarashi K, Suzuki O, Hara Y, Yoshiki Y, Okubo K (1998) Comparison of the protective effects of epigallocatechin gallate and epigallocatechin on paraquat-induced oxidative stress in rats. Food Sci Technol Int Tokyo 4: 149-154.
- Ando N, Igarashi K, Takenaka A, Hara Y (2000) A comparison of the protective effects between epigallocatechin gallate or epicatechin gallate and the mixtures of their components on paraquat-induced oxidative stress in rats. Food Sci Technol Res 6: 146-1499.
- Kim DW, Cho HI, Jun KM, Kim SJ, Choi JS, et al. (2012) Isorahamnetin-3-O-galactoside protects against CCl4-induced hepatic injury in mice. Biomol Ther (Seoul) 20: 406-412. [Crossref]
- Igarashi K, Ohnuma M (1995) Effects of isorhamnetin, rhamnetin, and quercetin on the concentrations of cholesterol and lipoperoxide in the serum and liver and on the blood and liver enzyme activities of rats. Biosci Biotechnol Biochem 59: 595-601. [Crossref]
- Wang C, Li K, Zhang YX, Shu KG, Chen XJ (2013) Effects of quercetin solid dispersion on oxidative stress of carbon tetrachloride inducing acute hepatic injury in mice. Xiandai Yufang Yixue 40: 2872-2874.
- Cui Y, Han Y, Yang X, Sun Y, Zhao Y (2014) Protective effects of quercetin and quercetin-5',8-disulfonate against carbon tetrachloride-caused oxidative liver injury in mice. Molecules 19: 291-305. [Crossref]
- Tsuchiya T, Suzuki O, Igarashi K (1996) Protective effects of chlorogenic acid on paraquat-induced oxidative stress in rats. Biosci Biotechnol Biochem 60: 765-768. [Crossref]
- Arlorio M, Locatelli M, Travaglia F, Coisson JD, Grosso ED, et al. (2008) Roasting impact on the contents of clovamide (N-caffeoyl-L-DOPA) and the antioxidant activity of cocoa beans (Theobroma cacao L.). Food Chem 106: 967-975.
- Zamperone A, Pietronave S, Colangelo D, Antonini S, Locatelli M, et al. (2014) Protective effects of clovamide against H2O2-induced stress in rat cardiomyoblast H9c2 Cell line. Food Funct 5: 2542-2551. [Crossref]
- Fallarini1 S, Miglio G, Paoletti1 T, Minassi1 A, Amoruso A, et al. (2009) Clovamide and rosmarinic acid induce neuroprotective effects in in vitro models of neuronal death. Br J Pharmacol 157: 1072–1084. [Crossref]
- Nomoto D, Tsunoda T, Shigemori H (2021) Effects of clovamide and its related compounds on the aggregations of amyloid polypeptides. J Nat Med 75: 299-307. [Crossref]
- Tsunoda T, Takase M, Shigemori H (2018) Structure-activity relationship of clovamide and its related compounds for the inhibition of β aggregation. Bioorg Med Chem 26: 3202-3209. [Crossref]
- Ley JP, Bertrm HJ (2007) Synthesis of lipophilic clovamide derivatives and their antioxidative potential against lipid peroxidation. J Agric Food Chem 51: 4596–4602. [Crossref]
- Correia AMNG, Leitao MCA, Clifford MN (1995) Caffeoyl-tyrosine and Angola II as characteristic markers for Angolan robusta coffees. Food Chem 53: 309-313.
- Tebayashi S, Ishiharaa A, Tsuda M, Iwamura 0048 (2000) Induction of clovamide by jasmoic acid in red clover. Phytochem 54: 387-392.
- Suda I (2000) Kosanka Kinou in “Shokuhin Kinou Kenkyuho” (in Japanese), eds. Shinohara K, Suzuki T, Kaminogawa S, Korin, Tokyo pp 2008-2020.
- Sugawara T, Igarashi K (2009) Identification of flavonoids in petals of edible chrysanthemum flowers and their suppressive effects on carbon tetrachloride-induced liver injury in mice. Food Sci Technol Res 15: 499-506.
- Reeves PG, Nielsen FH, Jr Fahey GC (1993) AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition Ad Hoc Writing Committee on the reformulation of the AIN-76A rodent diet. J Nutr 123: 1939-1951. [Crossref]
- Matsumoto K (1955) Transaminase, in “Protein, Nucleic Acid and Enzyme” An extra No. 24 ’81-11 (in Japanese), 94-96.
- Spencer JPE, Schroeter H, Crossthwaithe AJ, Kuhnle G, Williams RJ, Rice-Evans C (2001) Contrasting influences of glucuronidation and O-methylation of epicatechin on hydrogen peroxide-induced cell death in neurons and fibroblasts. Free Radical Biol Med 31: 1139–1146. [Crossref]
- Spencer JPE, Schroeter H, Kuhnle G, Srai S K, Tyrrell RM, et al. (2001) Epicatechin and its in vivo metabolite, 3-O-methyl epicatechin, protect human fibroblasts from oxidative-stress-induced cell death involving caspase-3activation. Biochem J 354: 493–500. [Crossref]
- Spencer JPE, Kuhnle GGC, Williams RJ, Rice-Evans C (2003) Intracellular metabolism and bioactivity of quercetin and its in vivo Metabolites. Biochem J 372: 173–181. [Crossref]
- Nakazawa T, Ohsawa K (1998) Metabolism of rosmarinic acid in rats. J Nat Prod 61: 993-996. [Crossref]
- Vareed SK, Kakarata M, Ruffin MK, Crowell JA, Normolle DP, et al. (2008) Pharmacokinetics of curcumin conjugate metabolism in healthy human subjects. Cancer Epidemiol Biomarkers Prev 17: 1411-1417. [Crossref]
- Stalmach A, Mullen W, Barron D, Uchida K, Yokota T, et al. (2009) Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: Identification of biomarkers of coffee consumption. Drug Metab Dispos 37: 1749-1758. [Crossref]
- Shimoi K, Okada H, Furugori M, Goda T, Takase S, et al. (1998) Intestinal absorption of luteolin and luteolin 7-O-β-glucoside in rats and humans. FEBS Lett 438: 220-224. [Crossref]
- Day AJ, Mellon F, Barron D, Sarrrazin G, Morgan MR, et al. (2001) Human Metabolism of dietary flavonoids: identification of plasma metabolites of quercetin. Free Radic Res 35: 941-952. [Crossref]
- Stark T, Lang R, Keller D, Hensel A, Hofmann T (2008) Absorption of N-phenylpropenoyl-L-amino acids in healthy humans by oral administration of cocoa (Theobroma cacao). Mol Nutr Food Res 52: 1201–1214. [Crossref]





