Abstract
Phenotypic, andrologic, and cytogenetic investigations were performed on a 2.5 years old buffalo-bull that produced azoospermic semen from puberty. Also, ultra-sonographic and histopathologic examinations were conducted on its testis. Phenotypic examinations showed atypical male features. Andrological investigations revealed small non-pendulous scrotum and reduced testicular size as indicated by lowered scrotal circumference measurement and ultrasound-detected testicular breadth. Both libido and serum testosterone concentration were greatly reduced. The sonographic examination of the test is revealed hypoechoic feature and reduced testicular breadth. Testicular hypoplasia was confirmed by the histopathological findings represented by a marked reduction in the number and diameter of seminiferous tubules and absence of mitotic or meiotic activities. Cytogenetic examination of peripheral leucocytes revealed the presence of trisomy-24.
It could be concluded that identifying trisomy -24 as possible cause of testicular hypoplasia in buffalo bulls is being useful for earlier diagnosis of the affected cases before reaching the age of puberty when the condition could be diagnosed and thus reducing the costs of keeping them till puberty.
Introduction
Keeping buffalo in small numbers among large number of breeders and wide spread use of uncontrolled natural service interfere with its genetic improvement and tracing up of genetic causes of infertility. Farm animals with spermatogenic impairment are usually not identified until the age they are expected to breed [1]. Among the non-infectious causes, genetic defects constitute an important factor for reproductive failure in cattle [2]. Cytogenetic studies in cattle, horses, sheep and pigs have been clearly identified chromosomal aberrations as one of the important factors contributing to infertility problems [3]. In cattle, testicular hypoplasia has been associated with chromosomal aberration such as mosacism [4] or 61xxy; the counterpart of human Klinefelter’s syndrome [5]. Abnormal distribution of sex chromosomes or autosomes bearing sex influencing genes may affect the development of the testis and male masculinity [6]. Trisomy of chromosomes-22 results in sterility and complete spermatogenic arrest in man [7]. In contrast to human, chromosomal aberrations in domestic animals may be more common than they appear probably because of lack of cytogentic investigators, facilities or difficulty in obtaining materials containing chromosomal aberrations [8]. The main aim of this work was to describe testicular hypoplasia in a buffalo bull and investigate its possible genetic cause.
Material and method
The animal and its phenotype
The buffalo bull used in the current study was born as singleton for two apparently normal parents of Egyptian buffalo; it was 2.5 years in age and weighed 500 kg. The bull has been recorded to ejaculate azoospermic semen from puberty. Five bulls having the similar age and body weight were used as a control group. All were reared in Mahallet Mousa Buffalo Experimental Station. The secondary sex characters of the affected bull were studied in comparison with control group.
Sexual desire and semen evaluations
Libido was assessed by estimation of reaction time. Semen was collected by means of artificial vagina twice weekly for 6 weeks. The collected semen was directly evaluated [9].
Serum testosterone concentration
Blood samples were collected once weekly for 6 weeks from the affected and control bulls. Sera were obtained by centrifugation (3000 rpm) for 10 minutes and stored at -20oC until assessment of testosterone.Serum testosterone concentrations were measured by Enzyme-linked immunosorb- antassay kits (ELISA).
Scrotal circumference (SC)
Scrotal circumferences for the affected bull and control ones were measured at the largest diameter of the testis according to Almiquist et al., [10].
Ultrasound examination of testis
The breadth and ultrasound features of the testis were detected by ultrasound scanning of each testes, while the animal was controlled in lateral recumbent position and one of its hind limb was pulled to the anterior and the other to the posterior. The ultrasound examination was done using a real time B-mode scanner (Ultrascan, 900, Alliance, Inc., Quebec, Canada) equipped with a 5 MHz linear transducer (L118 x D23 mm).
Histopathological examination
At slaughter, the testis of the affected bull and one of the control bulls were dissected, photographed and weighed. Several sections were taken from the core, peripheral parts of the 2 poles and middle part of the testis and fixed for histopathological examination [11].
Cytogenetic examination
Blood samples were collected from jugular vein by means of 5 ml heparinized vacutainer tubes. Leucocytes were cultured according to the modified method reported by Basrur and Gilman [12]. Air and flame dried slides were prepared. Some were stained using Geimsa stain 10%, while others were treated for G-banding technique according to Seabright, [13]. The stained slides were examined for metaphase plates. Suitable plates were examined and photographed for preparation of karyotypes.
Result
Phenotypic characters
The affected bull showed atypical male phenotype. Its face shifted to the female type when compared with that of a normal bull (Figure 1). Unlike those of the normal, the scrotum of the affected bull was not pendulous in hot weather (Figure 2).
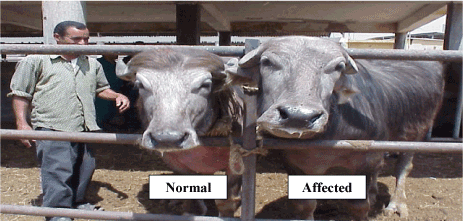
Figure 1. Faces of both normal and affected bulls showing the feminine characters on the face of the affected bull.

Figure 2. Scrotum of both affected and normal bulls showing non-pendulous scrotum in the affected bull.
Sexual desire and semen characteristics
The affected bull had a reduced sexual desire as indicated by mounting after hesitation with feeble holding and longer reaction time compared with normal bulls (Table 1). At all times of semen collections, the ejaculate of the affected bulls was clear, watery and azoospermic. The overall mean of the ejaculate volume of the affected bull was greatly reduced compared with that of the controls (Table 1).
Table 1. The reaction time, semen characteristics and serum testosterone concentration in the affected and normal control bulls.
Group
Parameters |
Affected bull |
Normal bulls (n=5) |
Reaction time (sec)
Ejaculate volume (ml)
Individual motility (%)
Sperm cell concentration (x106/ml)
Serum testosterone concentration (ng/ml) |
564 ±15.1
1.8±0.39
ND
ND
0.13±0.2 |
56.9±8.5
3.1±0.23
79.8±4.8
1127±48.17
0.89±0.6 |
Serum testosterone concentration
The overall mean of the serum testosterone concentration in the affected bull was lower than that in normal bulls (Table 1).
Scrotal circumference and ultrasound-detected testicular breadth
The overall means of scrotal circumferences were 20.5 cm and 32.8 cm for the affected and a normal comparable bull, respectively. The weights and ultrasound-detected testicular-breadths of both testes in the affected bull were numerically lower than those of the normal ones (Table 2 and Figure 3).
Table 2. The scrotal circumferences, testicular breadths and weights for the affected bull and a normal control one.
Group |
Ultrasound detected testicular-breadth |
Scrotal circumference
(SC)(cm) |
Testicular weight (gm) |
Right testes (mm) |
Left testes (mm) |
Right testes |
Left testes |
Affected bull
Control bull
Ratio |
17.9
53.4
1/3.0 |
18.3
43.4
1/2.8 |
20.5
32.8
1/1.6 |
32.3
203.4
1/6.3 |
36.2
196.8
1/5.5 |
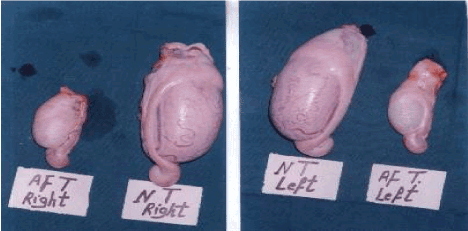
Figure 3. Photograph of the right and left testis in both affected (AF) bull and (NT) normal control one.
Ultrasonographic features
The testes of one of the normal control bulls appeared moderate and homogenous echogenic with a central hyperechoic line, representing mediastinum testis while that of the affected one appeared hypoechoic with hyperechoic central line (Figure 4).
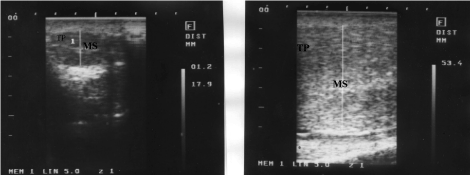
Figure 4. Ultrasonographic feature of affected (a) and normal testes (b)
Histopathological findings
Histopathological examination of the testes of the affected bull revealed a marked reduction in the number of the semniferous tubules with massive interstitium in-between (Figure 5b) compared with that of the normal bull (Figure 5a). The interstitium is formed from large amount of collagen fibers and numerous blood vessels and islets of Leydig cells with some of them containing pyknotic nuclei (Figure 6a). Also, the diameters of semniferous tubules were markedly reduced with no evidence of spermatogenesis as indicated by absence of both mitotic and meoitic activities (Figure 6b). They were lined by only one layer of spermatogonia. In contrast to the normal (Figure 5a), the somniferous tubules were collapsed or folded (Figure 5b).
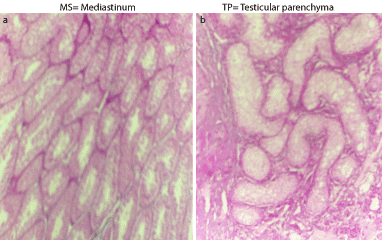
Figure 5. Cross section in the testes of the normal (a) and affected (b) bulls stained with H&E (x100).
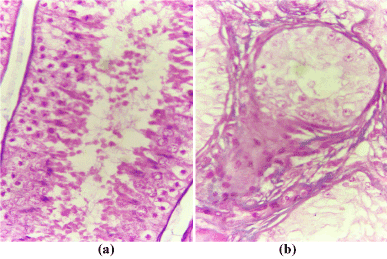
Figure 6. Cross section in the testes of the normal (a) and affected (b) bulls stained with H&E (x 400).
Cytogentic findings
Examination of Geimsa stained metaphase spreads of the affected bull revealed four lines of cells: 50, xy/48, xy/49, xy/ and 51, xy with 76 (65.5%)/9 (7.8%) / 15 (12.9%) and 17 (14.7%), respectively. The normal Geimsa stained karyotype showed that the extra chromosome was identified as chromosome 24. The same results were obtained after G-banding study (Figure 7).
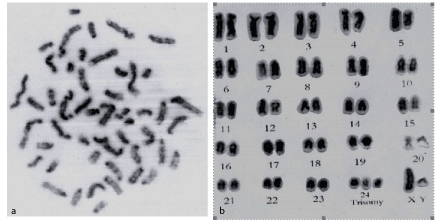
Figure 7: G- banded metaphse (a) and karyotype (b) of 51, xy buffalo bull with testicular hypoplas
2021 Copyright OAT. All rights reserv
Discussion
The reduction in the testicular size may be attributed to non-genetic factors such as testicular degeneration or chronic orchitis [14] or genetic affections such as testicular hypoplasia [15].
As reported in previous studies [14,16,17], the bilateral marked reduction in both testicular size and weight together with production of azoospermic semen from the age of puberty recorded in the present study suggest that the present condition might be bilateral complete testicular hypoplasia. Such suggestion may be supported by the findings of Moura and Erickson [1] who found that the difference in the testicular weight and seminiferous tubules diameter between normal and bull affected with testicular hypoplasia was mainly due to absence of the germ cells. This suggestion was confirmed by the histopathological findings where there was few numbers of the seminiferous tubules that were lined with one layer of the spermatogonia with absence of any mitotic and meiotic activities. These findings are similar to those reported by Bongso et al., [2]. In contrast to the moderate homogenous echogenic feature with central hyperechoic line characteristic of the normal testes [17] in the control bull, the testes of the affected bull showed a relative hypoechoic feature. However, the hypoechoic feature may be explained in the light of marked reduction in the number of the semniferous tubules and presence of one layer of spermatogonia within the tubules.
The atypical male features of the affected bull may be attributed to the abnormal distribution of the autosome-24 that might be a one of those bearing masculinity determining genes. Pineda [6] cited that genes for masculinity on both autosomes and on Y- chromosome must overcome and prevail over the female determining genes on the single x-chromosomes of an XY individual. However further studies are needed to determine if such chromosome is carrying the concerned gene.
Also, the lower serum testosterone concentration in the bull with testicular hypoplasia compared with normal bulls explains the presence of both feminine characters and low libido. Such low serum testosterone concentration may be attributed to the lower rate of testosterone production by the hypoplastic testes compared with the normal ones [18]. Veeramachaueni et al., [19] attributed the lowered serum testosterone concentration in bulls with testicular hypoplasia to the impairment in the function of the Leydig's cells by local factors either causing tubular damage or those consequent to the tubular damage.
Identification of trisomy in the autosome 24 in affected bull suggests a correlation between testicular hypoplasia and such chromosomal aberration. According to the standard Karyotype of the river buffalo [20,21] this chromosome resembles chromosome 25 in cattle. Chromosome number 25 in cattle carrying many genes some of them are reported to play a role in fertility.
Testicular organogenesis involves a cascade of gene activation and differentiation of the component cell types [22]. When cell organization is abnormal, the testes is described as dysgenetic, which could be resulted from chromosomal abnormalities [23].
According to the available literature this may be the first record to correlate between testicular hypoplasia and trisomy-24 in buffalo bulls. A comparable study in human, reported that trisomy-21 resulted in sterility and complete spermatogenetic arrest in man [7]. However, Shah et al. [7] attributed such condition to the reduced proliferations of primordial germ cells during migration to the gonadal ridge and accelerated rate of appotitosis.
It could be concluded that identifying trisomy of autosomal chromosome 24 as a possible cause of testicular hypoplasia in buffalo bulls may aid in elimination of the affected cases in young ages and not wait the age of puberty when the semen could be collected, and the affection could be diagnosed.
References
- Moura AA, Erickson BH (2001) Testicular development, histology and hormone profiles in three yearling angus bulls with spermatogenic arrest. Theriogenology 55: 1469-1488. [Crossref]
- Bongso TA, Jainudeen MR, Lee JY (1981) Testicular hypoplasia in a bull with XX/XY chimerism. Cornell Vet 71: 376-382. [Crossref]
- Prakash B, Balain DS, Lathwal SS, Malik RK (1994) Trisomy-X in a sterile river buffalo. Vet Rec 134: 241-242. [Crossref]
- Lodija L,Rubes J, Stavkova M, Havrankova J (1976) Chromosomal finding in some reproductive disorders in bull. Proc. 18th Int. Cong. Anim. Reprod and A. I. Krakow, I.:158.
- Dum HO, Lein DH, McEntee K (1980) Testicular hypoplasia in a Hereford bull with 61xxy karyotype: the bovine counterpart of human klinefelter’s syndrome. Cornell Vet 70: 137-146. [Crossref]
- Pineda MM (2003) The biology of sex. In: McDonald’s Veterinary Endocrinology and Reproduction. 5th ed. Iowa State press.
- Shah K, Sivapalan G, Gibbons N, Tempest H, Griffin DK (2003) The genetic basis of infertility. Reproduction 126: 13-25. [Crossref]
- Bhatia S, Shanker V (1992) First report of a XX/XXY fertile goat buck. Vet Rec 130: 271-272. [Crossref]
- Salisbury GW, Vandemark NK, Lodge JR (1979) Physiology of reproduction and artificial insemination in cattle. 2nd ed. W.H. Freeman and Co. San, Francisco, USA.
- Almiquist JO, Branas RJ, Barber KA (1976) Post-pubertal changes in semen production of charolias bulls ejaculated at high frequency and the relation between testicular measurements and sperm output. J Anim Sci 42: 670.
- Drury RAB, Willington E B (1980) Carton Histological Techniques. 5th Ed. PP. 139-307. Oxford Univ, Press, England.
- Basrur PK, Gilman JPW (1964) Blood culture method for the study of bovine chromosomes. Nature 204: 1335-1337. [Crossref]
- Seabright M (1971) A rapid banding technique for human chromosomes. Lancet 2: 971-972. [Crossref]
- Robert’s J (1986) Veterinary obstetrics and genital diseases. Edited by the authors.
- Galloway DE, Norman JR (1979) Testicular hypoplasia and autosomal/secondary contractions in bulls. Proc. VIII Int. Congr. Anim. Reprod. Artif. Insem., Krakow, IV, 710.
- Kirshnalingam V, Ladds PW, Entwistle KW, Holroyd RG (1982) Quantative macroscopic and histological study of testicular hypoplesia in Bos indicus strain bulls. Res Vet Sci 32: 131-139. [Crossref]
- Pechman RD, Eilts BE (1987) B-mode ultrasonography of the bull testicle. Theriogenology 27: 431-441. [Crossref]
- Kay GW, Grobbelaar JA, Hattingh J (1992) Heritable testicular hypoplasia in Nguni (Bos indicus) bulls: vascular characteristics and testosterone production. J Reprod Fertil 96: 537-547. [Crossref]
- Veermachaneni DN, Ott,RS, Heath RS, McEntee,K, Blott, DJ, et al. (1986) Pathophysiology of small testes in beef bulls: relationship between scrotal circumference, histopathologic features of testes and epididmyes, seminal characteristics and the endocrine profile. Anim J Vet Res 47: 1988-1999. [Crossref]
- Iannuzzi L (1994) Coordinator for the; standard karyotype of the river buffalo (Bubalus bubalis L., 2n=50). Report of the committee for the standardization of banded karyotypes of the river buffalo. Cytogenet cell Genet 67: 102-112.
- ISCNDB (2000) International system for Chromosome Nomenclature of Domestic Bovids. Editors: D. DiBerardino, G. P. Di Meo, D. S. Gallagher, H. Hayes and L. Iannuzzi. Cytogenet cell Genet 92: 283-299.
- Fisher JS, Macpherson S, Marchetti N, Richard MS (2003) Human testicular dysgenesis syndrome: a possible model using in-utero exposure of the rat to dibutyl phthalate. Human Reprod 18: 1383-1394. [Crossref]
- Skakkebaek NE, Rajpert-De Meyts E, Main K (2001) Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 16: 972-978. [Crossref]







