The present study was conducted to evaluate the ovarian potential of 87 local ewes from Ngaoundere, Adamawa region (Cameroon) for in vitro oocyte production. The ovaries were excised, submerged in normal saline solution (0.9%) and transported to the laboratory for a detailed evaluation. Follicles on each ovary were counted, their diameters (Φ) measured and were grouped into 3 categories: small (Φ <2 mm), medium (2 ≥ Φ ≤ 4 mm) and large (4> Φ ≤ 10 mm). Each ovary was then sliced into a petri dish; the oocytes were recovered in Dulbecco’s phosphate buffered saline, examined under a stereoscope (x10) and graded into four groups based on the morphology of cumulus oophorus cells and cytoplasmic changes of the oocytes. The effects of both ovarian (ovarian localization, corpus luteum, size and weight of ovary) and non-ovarian factors (breed, age, body condition score (BCS) and pregnancy status) on the follicular population and oocyte recovery rate were determined. The average follicular population was 8.80 ± 2.97 follicles per ovary. The number of small, medium and large follicles were 6.13 ± 2.90; 1.60 ± 0.49 and 1.06 ± 0.05, respectively. Oocyte recovery rate were 6.04 ± 1.01 per ovary. Oocytes graded I, II, III and IV were 1.20 ± 0.18 (11%); 1.23 ± 0.23(14%); 1.13 ± 0.12 (7%)and 2.47 ± 0.93 (68%), respectively. The oocyte quality index was 1.98. Younger, non-pregnant ewes with a body condition score of 3 and large ovaries presented higher number of follicles and oocytes. Oocytes (grades I and II) acceptable for in vitro embryo production (IVEP) constituted 25% of the harvest. This study indicates that some factors such as age, body condition score, pregnancy status, ovarian localization, weight of ovary and corpus luteum must be taken into account to increase the potential of the ovary for IVEP.
native ewes, follicular population, oocyte quality, ovary
In Cameroon, the number of small ruminants is estimated at around 7 million heads, including 3.2 million sheep [1]. The sheep breeds encountered are among others the Djallonke, fulani of the Wayla and Ouda varieties, Mayo Kebbi and Kirdi. They are commonly called "the poor man’s meat" [2]. However, this breeding is confronted with many problems in particular the reproduction remaining natural without any assistance of biotechnologies in particular artificial insemination, in vitro fertilization and embryo transfer [3]. The advantage of using these reproductive biotechnologies, is that it considerably increases the size of the herd while contributing to the preservation of the genetic heritage of sub fertile or dead animals [4].
Indeed, the ovaries can be considered as a sustainable and inexpensive source in which can be used to study the development and application of biotechnology of reproduction, conservation and cell freezing [5]. Embryos can be produced and transferred to recipient females from oocytes collected from the ovaries of slaughtered females. However, the development and application of assisted reproductive technologies such as in vitro embryo production (IVEP) through in vitro maturation (IVM), in vitro fertilization (IVF) and in vitro culture can revolutionize research on domestic livestock [6]. In addition, the production of a good embryo necessarily requires better quality oocytes [7]. The initial and most important step in in vitro fertilization is the selection of viable oocytes capable of being matured in vitro [8]. In sub-Saharan Africa, the recovery rate of oocytes is low and the cost of embryo production in vitro is high [9]. To our knowledge, no study has been done on the characterization of follicles and oocytes of native ewes in Cameroon. Consequently, the present study was carried out with the aim of determining the ovarian potential of the local sheep for the production of oocytes in vitro in Cameroon. Specifically, we will determine the follicular population and oocytes recovery rate; assess the influence of ovarian and non-ovarian parameters on follicular population, yield and quality of oocytes.
Study area
The present study was carried out using a samples of ewes taken at the small ruminant slaughterhouse Bantaϊ and analyzed at the biochemical analysis laboratory (ADAM’S LABO) in the Adamawa region of Cameroon. Ngaoundere, chief town of the Adamawa region is situated between Latitude 7°19'39N and Longitude 13°35'4E and have an average annual rainfall of 1496.7 mm. The temperatures varied from 15.2°C to 29°C with an average humidity of 58.2%. The study was conducted from November 2019 to January 2020. The slaughtered ewes were from the Vina Division (62.1%), Mayo Rey Division (31%) and Mbam and Kim (6.9%).
Characteristics of animals
A total of 87 local ewes of different breeds [Djallonke (67), Peul (8), Kirdi (8) and Mayo Kebbi (4)] were selected for this study. The average live weight of the animals was measured using a Mechanical Pocket Balance 100Kg of precision 1 Kg. The body condition score (BCS) and age were determined as described by Russel et al. [10] and Salami [11], respectively. For pregnant ewes, the fetal age was determined by the formula X = 2.1 (y + 17), y represented the cranio-caudal length in cm and X the duration of pregnancy in days [12] and the length of pregnancy was classified in three groups: ≤ 50 days, 51-100 days and >100 days.
Ovary collection and handling
After slaughter, the left and right ovaries were removed using scissors and placed in separate conical tubes containing Washed Medium (WM) and transported to the laboratory at 35-37°C within next 2 hours after slaughter. All cystic ovaries (Φ of the follicles >10 mm) were excluded from studies [13].
Determination of the weight and the size of the ovary
In the laboratory, the ovaries were removed from the tubes and placed in a petri dish, excessive tissues attached to the ovaries were carefully trimmed off and ovaries were weighed using an electronic Digital Scale with a precision of 0.01. The length, width and thickness of the ovaries were measured using a digital caliper like Carbon Fiber Composites Digital Caliper and the ovaries were thereafter allocated into two size groups (<1.70x 1.26x 0.75 and >1.70x 1.26x 0.75). Three types of corpus luteum have been identified: white, hemorrhagic and yellow.
Determination of follicular population
The ovaries were washed with washing medium. For each ovary, visible follicles were counted and follicular diameters (Φs) was measured with digital caliper. Follicular Φs were classified into 3 categories: small (<2 mm), medium ([2 to 4] mm) and large (]4 to 10] mm) as described by Mohamed et al. [14].
Recovery and grading of oocytes
The ovaries were placed in separate plastic Petri dishes containing Dulbecco’s Phosphate Buffered Saline (DPBS) and the slicing technique was used to collect oocytes [13]. Oocyte quality was evaluated under a stereoscope (x10) and scored into four grades (G) according to the homogeneity of the cytoplasm and layers of cumulus cells as described by Khandoker et al. [15]. Grade I (GI): Oocytes with more than 4 layers of bunch of compact cumulus cells mass with evenly granulated cytoplasm; grade II (GII): oocyte with at least 2–4 layers of compact cumulus cell mass with evenly granulated cytoplasm; grade III (GIII): Oocyte with at least one layer of compact cumulus cell mass with evenly granulated cytoplasm; grade IV (GIV): Denuded oocyte with no cumulus cells or incomplete layer of cumulus cell or expanded cells and having dark or unevenly granulated cytoplasm. The overall quality was calculated as an index using the formula [(G I x 1 + G II x 2 + G III x 3 + G IV x 4) / Total number of oocytes recovered] as described by Baki Acar et al. [16]. Index values that approache one reflected good quality oocytes.
Statistical analysis
Data were analyzed using SPSS (Statistical Package for Social Sciences) Version 25.0. The analysis of variance and Duncan's test statistics were used to analyze appropriate data sets. Differences were significant at P <0.05.
Characteristics of slaughtered ewes
The mean live weight (Kg), BCS and age (years) of the ewes were 27.25 ± 4.16; 2.44 ± 0.54 and 2.71 ± 1.33, respectively. A pregnancy rate of 68% was recorded and the average age of the fetal was around 72 days.
The mean weight (g) of the ovaries was 1.16 ± 0.49. The right ovaries (1.18 ± 0.45 g) were heavier than the left (1.15 ± 0.55 g). The length, wide and thickness (cm) of the ovaries were 1.7 ± 0.25, 1.26 ± 0.18 and 0.75 ± 0.14, respectively. The ovaries of pregnant ewes were significantly (P<0.05) larger and heavier than those of non-pregnant ones (Table 1).
Table 1. Means ± SD values of the weight and size of the ovaries, breed, BCS, age, pregnancy status and corpus luteum
Parameters |
Variables |
N |
Left ovary weight (g) |
Right ovary weight (g) |
Ovary weight (g) per animal |
Ovary length (cm) |
Ovary width (cm) |
Ovary thickness (cm) |
Breed |
Djallonke |
67 |
1.13 ± 0.53ab |
1.19 ± 0.44a |
1.16 ± 0.38ab |
1.69 ± 0.24a |
1.27 ± 0.17b |
0.75 ± 0.14ab |
Kirdi |
8 |
0.78 ± 0.51a |
0.97 ± 0.46a |
0.87 ± 0.46a |
1.51 ± 0.18a |
1.07 ± 0.05a |
0.64 ± 0.06a |
Mayo Kebbi |
4 |
1.47 ± 0.30b |
0.95 ± 0.44a |
1.21 ± 0.34ab |
1.71 ± 0.16a |
1.20 ± 0.13b |
0.74 ± 0.10ab |
Peul |
8 |
1.52 ± 0.59ab |
1.48 ± 0.45a |
1.50 ± 0.41b |
2.01 ± 0.15b |
1.48 ± 0.17c |
0.85 ± 0.15b |
P-value |
|
0.045 |
0.095 |
0.039 |
0.001 |
0.000 |
0.019 |
BCS |
1 (Thin) |
2 |
1.60 ± 0.01a |
1.64 ± 0.05a |
1.62 ± 0.03a |
1.83 ± 0.04a |
1.35 ± 0.05a |
0.72 ± 0.11a |
2 (Medium) |
45 |
1.13 ± 0.54a |
1.18 ± 0.42a |
1.16 ± 0.39a |
1.71 ± 0.21a |
1.27 ± 0.19a |
0.73 ± 0.14a |
3 (Good) |
40 |
1.14 ± 0.57a |
1.16 ± 0.50a |
1.15 ± 0.42a |
1.68 ± 0.29a |
1.26 ± 0.18a |
0.78 ± 0.13a |
P-value |
|
0.235 |
0.320 |
0.224 |
0.521 |
0.590 |
0.083 |
Age (years) |
< 1 |
10 |
1.21 ± 0.31ab |
1.15 ± 0.29ab |
1.18 ± 0.17b |
1.74 ± 0.19b |
1.37 ± 0.21b |
0.77 ± 0.08a |
[1-2] |
14 |
0.78 ± 0.41a |
0.90 ± 0.36a |
0.84 ± 0.34a |
1.49 ± 0.20a |
1.13 ± 0.12a |
0.72 ± 0.10a |
[2-3] |
7 |
1.11 ± 0.65ab |
1.04 ± 0.41ab |
1.07 ± 0.47ab |
1.62 ± 0.21ab |
1.22 ± 0.14ab |
0.72 ± 0.12a |
[3-4] |
56 |
1.23 ± 0.57b |
1.28 ± 0.47b |
1.26 ± 0.41b |
1.76 ± 0.24b |
1.29 ± 0.18b |
0.76 ± 0.16a |
P-value |
|
0.034 |
0.028 |
0.005 |
0.005 |
0.006 |
0.657 |
Pregnancy status |
Non pregnant |
28 |
1.05 ± 0.44a |
0.99 ± 0.42a |
1.02 ± 0.41a |
1.64 ± 0.25a |
1.20 ± 0.20a |
0.72 ± 0.16a |
Pregnant |
59 |
1.19 ± 0.59a |
1.27 ± 0.44b |
1.23 ± 0.39b |
1.73 ± 0.24a |
1.30 ± 0.17b |
0.77 ± 0.13a |
P-value |
|
0.298 |
0.005 |
0.026 |
0.078 |
0.004 |
0.060 |
Corpus luteum |
Absent |
15 |
0.90 ± 0.44a |
0.98 ± 0.43a |
0.94 ± 0.41a |
1.61 ± 0.17a |
1.12 ± 0.17a |
0.65 ± 0.11a |
Present |
72 |
1.20 ± 0.56a |
1.23 ± 0.45b |
1.21 ± 0.39b |
1.72 ± 0.26a |
1.30 ± 0.17b |
0.77 ± 0.14b |
P-value |
|
0.064 |
0.047 |
0.028 |
0.131 |
0.000 |
0.002 |
a,b,c In each column different letters indicated significant difference between group (p<0.05)
N=number of ewes
SD=standard deviation
Follicular population
From 174 ovaries, 1,194 follicles were counted. The mean number of follicles per ovary recorded was 8.80 ± 2.97. Small (Φ ˂2mm), medium (2 ≥ Φ ≤ 4 mm) and large (4> Φ ≤ 10 mm) follicles represented 87%, 11% and 2% of the follicular population, respectively.
Yield and quality of oocytes
The average number of oocytes recovered per ovary was 6.04 ± 1.01 (n = 607) with a recovery rate of 65%. The quality of the oocytes graded I, II, III and IV (Figure 1) were 1.20 ± 0.18 (11%), 1.23 ± 0.23 (14%) 1.13 ± 0.12 (7%) and 2.47 ± 0.93 (68%), respectively. Selected oocytes for in vitro embryo production (IVEP) (G I and II) represented 2.43 ± 0.41 (25%) per ovary. The oocyte index was 1.98.
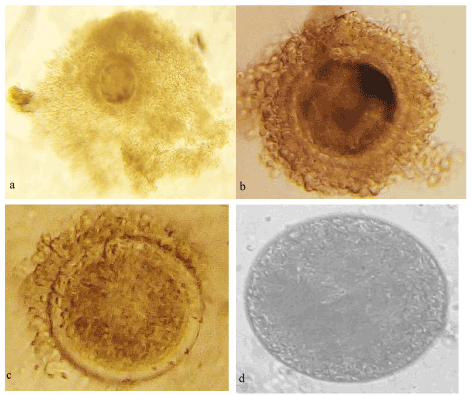
Figure 1. a= Oocyte grade I; b= Oocyte grade II; c= Oocyte grade III; d= Oocyte grade IV
Influence of ovarian parameters (ovarian localization, corpus luteum, size and weight of ovary) on the follicular population, number and grade of the oocytes
The right ovary tended to have more follicles than the left (P>0.05). However, the left ovary tended to have more oocytes (P>0.05). The total number of follicles increased with the weight (g) and size (cm) of the ovary; however, the yield and quality of the oocyte decreased with the weight and size of the ovary. On the other hand, ovaries with a corpus luteum had fewer follicles and oocytes acceptable for IVEP (Tables 2 and 3).
Table 2. Effects of ovarian factors on follicular population. a,b, In each column different letters indicated significant difference between group (p<0.05) N=number of ewes
Parameters |
Variables |
N |
Number of follicles |
Average number of follicles /ovary |
|
|
|
Small (<2 mm) |
Medium ([2-4] mm) |
Large (]4-10] mm) |
Ovary localization |
Left |
87 |
5.86 ± 3.03a |
1.45 ± 0.48b |
1.00 ± 0.00b |
8.28 ± 3.15a |
Right |
87 |
6.30 ± 3.91a |
1.72 ± 0.77a |
1.05 ± 0.21a |
9.06 ± 3.92a |
P-value |
|
0.420 |
0..007 |
0.085 |
0.148 |
Corpus luteum |
Absent |
15 |
6.07 ± 3.08a |
1.83 ± 0.20b |
1.00 ± 0.00a |
8.90 ± 3.08a |
Present |
72 |
6.15 ± 2.89a |
1.58 ± 0.53a |
1.07 ± 0.05b |
8.81 ± 2.98a |
P-value |
|
0.995 |
0.000 |
0.000 |
0.901 |
Ovary weight (g) |
<1 |
27 |
6.55 ± 3.47a |
1.50 ± 0.21a |
1.00 ± 0.00a |
9.05 ± 3.51a |
[1-1.50] |
41 |
5.73 ± 2.66a |
1.62 ± 0.52a |
1.00 ± 0.00a |
8.35 ± 2.77a |
>1.50 |
19 |
6.70 ± 2.38a |
1.71 ± 0.68a |
1.10 ± 0.11b |
9.51 ± 2.39a |
P-value |
|
0.340 |
0.146 |
0.000 |
0.185 |
Ovary size (cm3) |
<1.70x1.26x0.75 |
50 |
5.94 ± 2.84a |
1.64 ± 0.44b |
1.00 ± 0.00a |
8.58 ± 2.88a |
>1.70x1.26x0.75 |
37 |
6.39 ± 3.02a |
1.56 ± 0.57a |
1.07 ± 0.08b |
9.02 ± 3.13a |
P-value |
|
0.656 |
0.000 |
0.000 |
0.673 |
Table 3. Effects of ovarian factors on oocyte number and grade
Parameters |
Variables |
N |
Average number of oocytes /ovary |
Oocyte grades |
Selected oocytes for IVEP. I and II |
|
I |
II |
III |
IV |
Ovary localization |
Left |
87 |
6.09 ± 1.30a |
1.17 ± 0.24b |
1.42 ± 0.44b |
1.03 ± 0.11a |
2.46 ± 1.16a |
2.59 ± 0.49b |
Right |
87 |
5.90 ± 1.31a |
1.14 ± 0.22a |
1.11 ± 0.20a |
1.16 ± 0.25b |
2.49 ± 1.26a |
2.25 ± 0.29a |
P-value |
|
0.244 |
0.00 |
0.000 |
0.000 |
0.874 |
0.000 |
Corpus luteum |
Absent |
15 |
5.59 ± 0.72a |
1.27 ± 0.24b |
1.25 ± 0.23a |
1.00 ± 0.00a |
2.07 ± 0.75a |
2.52 ± 0.44a |
Present |
72 |
6.14 ± 1.06a |
1.19 ± 0.17a |
1.23 ± 0.24a |
1.17 ± 0.14b |
2.55 ± 0.96a |
2.42 ± 0.25a |
P-value |
|
0.170 |
0.003 |
0.055 |
0.000 |
0.051 |
0.193 |
Ovary weight (g) |
<1 |
27 |
6.20 ± 1.24a |
1.16 ± 0.09b |
1.38 ± 0.23b |
1.00 ± 0.00a |
2.66 ± 1.12a |
2.54 ± 0.24c |
[1-1.50] |
41 |
5.82 ± 0.84a |
1.30 ± 0.20c |
1.17 ± 0.21a |
1.13 ± 0.12b |
2.21 ± 0.83a |
2.47 ± 0.31b |
>1.50 |
19 |
6.46 ± 0.91a |
1.15 ± 0.21a |
1.21 ± 0.26a |
1.33 ± 0.19c |
2.76 ± 0.73a |
2.36 ± 0.31a |
P-value |
|
0.089 |
0.000 |
0.001 |
0.000 |
0.087 |
0.003 |
Ovary size (cm3) |
<1.70x1.26x0.75 |
50 |
6.15 ± 1.10a |
1.21 ± 0.19a |
1.25 ± 0.25b |
1.23 ± 0.16b |
2.46 ± 1.03a |
2.46 ± 0.32a |
>1.70x1.26x0.75 |
37 |
5.97 ± 0.89a |
1.26 ± 0.18b |
1.21 ± 0.22a |
1.00 ± 0.00a |
2.49 ± 0.79a |
2.47 ± 0.25a |
P-value |
|
0.614 |
0.000 |
0.000 |
0.000 |
0.908 |
0.916 |
a,b,c In each column different letters indicated significant difference between group (p<0.05)
N=number of ewes
Influence of non-ovarian parameters (breed, age, BCS, state and pregnancy length) on the follicular population, number and grade of the oocytes
The total number of follicles and oocytes was higher in ewes less than 3 years old and with a BCS of 3. However, non-pregnant ewes or those whose pregnant was found in the first three months presented also a large number of follicles and a good yield of oocytes (Tables 4 and 5).
Table 4. Effects of non-ovarian factors on follicular population
Parameters |
Variables |
N |
Number of follicles |
Average number of follicles /ovary |
|
Small (<2mm) |
Medium ([2-4] mm) |
Large (]4-10] mm) |
Breed |
Djallonke |
67 |
5.79 ± 2.80ab |
1.54 ± 0.43b |
1.00 ± 0.00a |
8.32 ± 2.90ab |
Kirdi |
8 |
9.25 ± 2.30c |
0.75 ± 0.19a |
1.00 ± 0.00a |
11.00 ± 2.35b |
Mayo Kebbi |
4 |
3.38 ± 1.75a |
2.00 ± 1.35b |
1.50 ± 0.00b |
6.88 ± 1.60a |
Peul |
8 |
6.69 ± 3.31bc |
1.63 ± 0.38b |
1.00 ± 0.00a |
9.31 ± 3.31ab |
P-value |
|
0.003 |
0.000 |
|
0.049 |
BCS |
1 |
2 |
8.50 ± 1.41a |
0.00 ± 0.00a |
0.50 ± 0.00a |
9.00 ± 1.41a |
2 |
45 |
6.12 ± 3.29a |
1.70 ± 0.59b |
1.17 ± 0.06b |
8.99 ± 3.28a |
3 |
40 |
5.73 ± 2.83a |
1.44 ± 0.33b |
1.00 ± 0.00c |
8.16 ± 2.94a |
P-value |
|
0.098 |
0.000 |
0.000 |
0.658 |
Age (years) |
<1 |
10 |
6.00 ± 2.52a |
2.19 ± 0.65b |
0.50 ± 0.00b |
8.69 ± 2.56ab |
[1-2] |
14 |
7.50 ± 3.28a |
1.24 ± 0.20a |
1.00 ± 0.00c |
9.74 ± 3.34b |
[2-3] |
7 |
5.50 ± 3.34a |
1.50 ± 0.29a |
0.00 ± 0.00a |
7.00 ± 3.48a |
[3-4] |
56 |
5.83 ± 2.87a |
1.48 ± 0.45a |
1.10 ± 0.06d |
8.41 ± 2.92ab |
P-value |
|
0.132 |
0.000 |
0.000 |
0.246 |
Pregnancy status |
Non pregnant |
28 |
6.90 ± 3.10a |
1.56 ± 0.43a |
0.72 ± 0.08a |
9.18 ± 3.24a |
pregnant |
59 |
5.78 ± 2.76a |
1.64 ± 0.53a |
1.10 ± 0.06b |
8.52 ± 2.81a |
P-value |
|
0.140 |
0.028 |
0.000 |
0.403 |
Pregnancy length in days |
[1-50] |
19 |
6.26 ± 3.28a |
1.51 ± 0.38b |
1.00 ± 0.00a |
8.78 ± 3.51a |
[51-100] |
27 |
5.62 ± 2.72a |
1.86 ± 0.68c |
1.13 ± 0.08b |
8.61 ± 2.72a |
>100 |
13 |
5.38 ± 1.84a |
1.00 ± 0.00a |
1.00 ± 0.00a |
7.38 ± 1.84a |
P-value |
|
0.374 |
0.000 |
0.000 |
0.172 |
a,b,c,d In each column different letters indicated significant difference between group (p<0.05)
N=number of ewes
Table 5. Effects of non-ovarian factors on oocytes number and grade
Parameters |
Variables |
N |
Average number of oocytes/ovary |
Oocyte grades |
Selected oocytes for IVEP. I and II |
|
|
|
I |
II |
III |
IV |
Breed |
Djallonke |
67 |
5.92 ± 1.01a |
1.22 ± 0.19b |
1.21 ± 0.23ab |
1.08 ± 0.08b |
2.40 ± 0.95a |
2.44 ± 0.30b |
Kirdi |
8 |
6.62 ± 0.89a |
1.00 ± 0.00a |
1.00 ± 0.00a |
1.25 ± 0.32c |
3.37 ± 0.83b |
2.00 ± 0.00a |
Mayo Kebbi |
4 |
4.54 ± 0.63b |
1.00 ± 0.00a |
1.00 ± 0.00a |
0.66 ± 0.23a |
1.87 ± 0.48a |
2.00 ± 0.00a |
Peul |
8 |
6.11 ± 0.66a |
1.33 ± 0.12b |
1.37 ± 0.33b |
1.00 ± 0.00b |
2.50 ± 0.46ab |
2.61 ± 0.34b |
P-value |
|
0.00 |
0.022 |
0.00 |
0.020 |
0.00 |
0.008 |
BCS |
1 |
2 |
4.25 ± 0.35a |
0.50 ± 0.00a |
1.00 ± 0.00a |
0.00 ± 0.00a |
2.75 ± 0.35a |
1.50 ± 0.00a |
2 |
45 |
6.17 ± 0.35b |
1.61 ± 0.18b |
1.27 ± 0.24b |
1.15 ± 0.11b |
2.59 ± 1.00a |
2.43 ± 0.25b |
3 |
40 |
5.76 ± 0.98b |
1.14 ± 0.12b |
1.22 ± 0.24ab |
1.11 ± 0.15b |
2.30 ± 0.91a |
2.35 ± 0.28b |
P-value |
|
0.041 |
0.000 |
0.204 |
0.000 |
0.442 |
0.000 |
Age (years) |
<1 |
10 |
5.45 ± 0.72ab |
1.00 ± 0.00a |
1.00 ± 0.00a |
1.00 ± 0.00b |
2.45 ± 0.72a |
2.00 ± 0.00a |
[1-2] |
14 |
5.68 ± 1.17b |
1.27 ± 0.23b |
1.20 ± 0.15b |
0.50 ± 0.00a |
2.71 ± 1.05a |
2.47 ± 0.26b |
[2-3] |
7 |
5.83 ± 1.12b |
1.00 ± 0.00a |
1.42 ± 0.25c |
1.00 ± 0.00b |
2.42 ± 1.02a |
2.42 ± 0.25b |
[3-4] |
56 |
4.79 ± 1.00a |
1.22 ± 0.20b |
1.28 ± 0.26bc |
1.18 ± 0.15c |
2.42 ± 0.94a |
2.50 ± 0.31b |
P-value |
|
0.154 |
0.000 |
0.001 |
0.000 |
0.489 |
0.000 |
Pregnancy status |
Non pregnant |
28 |
6.04 ± 0.98a |
1.43 ± 0.24b |
1.25 ± 0.26a |
1.18 ± 0.20b |
2.17 ± 0.79a |
2.68 ± 0.37b |
Pregnant |
59 |
6.00 ± 1.06a |
1.04 ± 0.06a |
1.22 ± 0.23a |
1.13 ± 0.08a |
2.61 ± 0.98a |
2.26 ± 0.23a |
P-value |
|
0.852 |
0.000 |
0.518 |
0.045 |
0.067 |
0.000 |
Pregnancy length in days |
[1-50] |
19 |
6.22 ± 1.27b |
1.00 ± 0.00a |
1.29 ± 0.33b |
1.00 ± 0.00a |
2.92 ± 1.10b |
2.29 ± 0.33b |
[51-100] |
27 |
6.12 ± 0.98b |
1.23 ± 0.18b |
1.15 ± 0.15ab |
1.13 ± 0.08b |
2.62 ± 0.94ab |
2.38 ± 0.22b |
>100 |
13 |
5.36 ± 0.68a |
1.00 ± 0.00a |
1.08 ± 0.13a |
1.17 ± 0.12b |
2.12 ± 0.71a |
2.08 ± 0.13a |
P-value |
|
0.000 |
0.000 |
0.023 |
0.003 |
0.072 |
0.054 |
a,b,c In each column different letters indicated significant difference between group (p<0.05)
N=number of ewes
The characteristics of the breeds of ewes in this study indicate that a large number of Djallonke breeds were slaughtered, which is contrary to that observed by Kouamo et al. [17] at the municipal slaughterhouse in Maroua with a dominance of Fulani sheep. However, the average age of slaughtered ewes was slightly higher than those recorded by Benchaib [18] and Kouamo et al. [17]. Indeed, the average live weight of ewes (27.25 ± 4.16 kg) was higher than those reported by Ngona et al. [19] in the Democratic Republic of Congo (20.50 ± 4.96 kg) and Kouamo et al. [17] (23.00 ± 2.90 kg). The difference could be explained by the large number of pregnant ewes studied.
The pregnancy rate (68%) was higher than that observed by Manjeli et al. [20] at the Garoua and Maroua slaugterhouse (38.60 %); Benchaib [18] in Algeria (26.00 %); Nana et al. [21] in the city of Dschang (49%) and Kouamo et al. [17] at the municipal slaughterhouse in Maroua (45.30 %). On the other hand, this rate is lower than that of Pitala et al. [22] in Togo (80.10 %). The difference could be related to the study period and the breed [17]. As reported by Manjeli et al. [20]; Pitala et al. [22] in Togo and Nana et al. [21] in Cameroon, the majority of pregnant ewes slaughtered were within the first three months of gestation. This could be explained on the one hand by the fact that the slaughter of pregnant females is not only due to ignorance of the physiological state of the animals because their physical appearance clearly indicates their physiological condition; but more because of the socio-economic context of small ruminant farming in our country which is considered as a subsistence farming to resolve urgent needs whatever the physiological state of the animals [20]. In fact, by slaughtering pregnant females, humans attack reproduction at its root, by exterminating future reproducers [21].
The average weight of the ovaries (1.16 ± 0.49 g) was lower than that reported by Mohamed et al. [14] (1.30 ± 0.23 g) and higher than that of Islam et al. [23] (0.69 ± 0.01 g) in goats. This difference may be due to the effect of the breed. The average dimensions of the ovaries were slightly larger than those of Islam et al. [23] and Mohamed et al. [14]. This result shows that the presence of the corpus luteum had a positive effect on the dimensions of the ovary because the corpus luteum formed from the follicle which ovulated develops in all direction on the surface of the ovary. However, the difference between the left and right ovaries has also been reported by Islam et al. [23], Mohamed et al. [14] and Asad et al. [7]. In fact ovulation was more marked in the right ovary. This greater activity of the right ovary would be responsible for its weight.
The average number of follicles (8.80 ± 2.97) was higher than that of Mohamed et al. [14] (4.9 ± 0.89) and Wani et al. [24] (7.46 ± 0.14). However, the number of follicles with diameters less than 2 mm was higher than those with diameters greater than 2 mm as reported by Mohamed et al. [14]. The slicing technique allows the recovery of all oocytes present in all follicles regardless of their location on the ovarian cortex. The average oocyte yield per ovary was 6.04 ± 1.01oocytes. This value is higher than that observed by Wani et al. [24] (5.87 ± 0.08 oocytes) and Rameez et al. [6] (4.51 ± 0.25 oocytes) in goats in India. This difference may be due to the collection technique used. However, greater follicular activity was recorded in the right ovary, with a larger number of large follicles (P<0.05) [14]. The total number of follicles was higher in the right ovaries [14,25,26]. However, ovaries without a corpus luteum had a higher number of oocytes acceptable for IVEP compared to ovaries with a corpus luteum [7,14,27]. This could be due to limited follicular development because lutein cells occupy a significant part of the ovary [6]. It can also arise from the fact that the corpus luteum could inhibit the growth of follicles and increase their atresia [28].
The total number of follicles acceptable grade of oocytes was higher in the ovaries of non-pregnant ewes than others [29]; however, Eias et al. [25] obtained a high number of follicles with pregnant female animals in the buffalo.
In conclusion, this study indicated that the ovaries of the native ewes in Cameroon have a low potential to produce acceptable oocytes for IVEP. Future studies must take into account certain parameters such as BCS, age and physiological status to maximize success of IVEP.
- Minepia (2013) Direction des services vétérinaires. Rapport annuel du service Régional de la Protection Sanitaire de l’Adamaoua. Ngaoundéré.
- MacHugh DE, Bradley DG (2001) Origines génétiques du bétail: Les chèvres résistent à la tendance. Proc Académie nationale des sciences 98: 5382-5384.
- Tchouamo IR, Tchoumboué J, Thibault L (2005) Caractéristiques socio-économiques et techniques de l’élevage de petits ruminants dans la province de l’ouest du Cameroun. Tropicultura 23: 201-211.
- Deuleuze S, Pointhier J, Hanzen C (2009) Reproduction assistée dans l’espèce equine Collecte evaluation maturation et utilisation d’ovocytes équins. Ann Méd Vét 153: 22-30.
- Ciptadi G, Rahayu S, Putri AI, Karima HN, Budiarto A, et al. (2019 IOP Conference Series. Earth Environ Sci.
- Dar R, Razzaque W, Bhat A, Reshi I, Kose M, et al. (2017) Efficacy of different harvesting techniques on oocyte recovery from goat ovaries. Intern J Livestock Res 7: 181-185.
- Asad L, Rahman A, Hossainand M, Akter M (2016) Ovarian category, follicles and oocytes analysis of goat ovaries in view of in vitro production of embryos. Int J Anim Res.
- Kouamo J, Kharche SD (2014) Dose dependent effect of pregnant mare serum gonadotropin and human chorionic gonadotropin on in vitro maturation of goat oocyte. Indian J Anim Sci 84: 410-414.
- Kouamo J, Sow A, Leye A, Sawadogo GJ, Ouedraogo GA (2009) Amélioration des performances de production et de reproduction des bovins par l’utilisation de l’insémination artificielle en Afrique Sub-saharienne et au Sénégal en particulier: état des lieux et perspectives. RASPA 7: 139-148.
- Russel AJ F, Doney JM, Gunn RJG (1969) Subjective assessment of body fat in live sheep. J Agric Sci Camb 72: 451-454.
- Salami I (1990) Determination de l’ âge par la dentition chez les petits ruminants en milieu traditionnel au Senegal. Thèse Med Vet EISMV Dakar.
- Arthur GH, Noakes DE, Pearson H (2001) Pregnancy and parturition: InVeterinary reproduction and obstetrics. Eighth Edition Bailliere Tindall, London.
- Wang ZG, Xu ZR, Yu SD (2007) Effects of oocyte collection techniques and maturation media on in vitro maturation and subsequent embryo development in boer goat. Czech J Anim Sci 52: 21-25.
- Mohamed AM, Alsafy KH, El-shahat (2011) Morphological evaluation of ovary in relation to recovery, quality of oocytes and steroid production in sheep. J App Biol Sci 5: 27-31.
- Khandoker M, Jahan N, Asad L, Hoque SAM, Ahmed S, et al. (2011) Qualitative et analyse quantitative des buffles les ovaires, les follicules et les ovocytes en vue de la production d'embryons in vitro. Coup J Anim Sci 40: 23-27.
- Baki Acar D, Birdane MK, Dogan N, Gurler H (2013) Effect of the stage of oestrus cycle on follicular population oocyte yield and quality, and biochemical composition of serum and follicular fluid in anatolian water buffalo. Anim Repro Sci 137: 8-14.
- Kouamo J, Obama SFF, Sassa MS (2019) Prevalence and risk factors of genital diseases of goats and ewes in the far north region of cameroun. PSM Vet Res 4: 62-73.
- Benchaib F (2007) Etude Comparative, descriptive et diagnostic de la pathologie génitale de la femelle des petits ruminants, Université d’Oran. Algérie.
- Ngona I, Bedium JM, Khang’Maté A, Hanzen C (2012) Etude descriptive des caractéristiques morphologiques et génitales de la chèvre de Lubumbashi en République démocratique du Congo. Rev Elev Med Vet Trop 65: 75-79.
- Manjeli Y, Njwe RM, Tchoumboué J, Abba S, Teguia A (1996) Evaluation des pertes d’agneaux et de chevreaux par abattage des femelles gravides. Revue Elev Med Vet Pays trop 49: 253-255.
- Nana FCN, Tume C, Daouda, Djitie FK, Dandji MBS, et al. (2014) Impact de l´abattage des chèvres gravides sur l´élevage des petits ruminants au Cameroun. Livest Res Rural Dev 26: 11.
- Pitala W, Arouna A, Kulo AE, Zongo M, Boly H, et al. (2012) Impact de l’abattage des brebis en gestation sur l’élevage au Togo. Livest Res Rural Dev 24: 11.
- Islam MR, Khandoker MAMY, Afroz S, Rahman MGM, Khan RI (2007) Qualitative and quantitative analysis of goat ovaries, follicles and oocytes in view of in vitro production of embryos. J Zhejiang University Sci 8: 465-469.
- Wani A, Khan MZ, Sofi KA, Malik AA, Lone F, et al. (2013) Effect of follicular size on in vitro maturation, fertilization and culture of sheep embryosIranian. J Vet Res 14: 299-304.
- Eias E, Osman I, Sharma RK, Majdi EB (2019) Determine the effect of ovarian and non-ovarian factors on follicular population in murrah buffalo using ultrasonography technique. Int J Vet Sci Anim Husb 4: 1-4.
- Majeed A, Omar A, Alrawi STJ, Maadhidy AFH, Ahmed KD, et al. (2019) Effect of collection methods on oocyte recovery rate in sheep. Res J Biotech 14: 262-264.
- Talukder MNS, Iqbal A, Khandoker MA M Y, Alam MZ (2011) Collection grading and evaluation of cumulus-oocytecomplexes for in vitro maturation in sheep. The Bangladesh Vet 28: 31-38.
- Hafez ESE(1993) Folliculogenesis, egg maturation and ovulation: Reproduction in farm animals. 5th Ed., Lea and Febiger, Phaildelphia, pp: 114-143.
- Kouamo J, Dawaze SM, Zoli AP, Bah GS (2014) Evaluation of bovine (Bos indicus) ovarian potential for in vitro embryon production in the Adamawa plateau (Cameroun). Open Vet J 4: 128-136.

