In the present study, two different reinforcement methods were utilized to improve the compressive mechanical properties of porous hydroxyapatite (HA) scaffold which normally exhibits brittle nature with low elastic modulus and strength. In the first method, biodegradable polymer PCL or PLLA was coated on the surface of HA base frame structure. On the other hand, in the second method, spongy porous structure of PCL or PLLA was created inside of the continuous pores of HA frame. Both the coated and 2-phase composite scaffolds showed dramatic improvement of compressive mechanical properties, mainly owing to the ductile deformation of the polymeric secondary phases. It is worth noting that the novel 2-phase scaffold exhibited greater improvement of mechanical properties than the coated one. It was also found that the 2-phase scaffold preserved the entire structure even after the localized fracture of the HA took place due to the supporting effect of spongy structure of polymer under compressive loading condition.
tissue engineering, Bone regeneration, Scaffold, composite material, Compressive properties, fracture mechanism
Reconstructive treatment of bone defects has been carried out widespread in orthopedic surgeries. Several major methods such as autografts (retrieved from the patient himself), allografts (obtained from same species or different species) and artificial bioceramic grafts made from calcium phosphates such as hydroxyapatite and - and -TCP [1,2]. Among of them, autografts have been considered as the ‘golden standard’ of bone grafting because it provides osteogenic cells as well as crucial osteoinductive factors required for bone regeneration [2-4], however, the capacity of use is restricted due to the limited amount of bone available in human body [3,5]. On the contrary, the availability of allografts is not restricted [6], however, there are some disadvantages such as the risk of pathogen transmissions including tumor cell transmission, bacterial and viral infection, and immune rejection [7,8]. Furthermore, costly bone banks must be prepared and maintained in order to supply such allografts continuously. To overcome those disadvantages, artificial engineering grafts have been developed by mainly using biocompatible calcium phosphates such as hydroxyapatite (HA), and - and - tricalcium phosphates ( - TCP and -TCP) because their chemical structures are very similar to carbonate apatite of bone tissue [9,10].
Recently, tissue engineering approach has also been considered in orthopedic surgery to regenerate large scale bone damages. In bone tissue engineering approach, there are three main components, i.e. cells, matrices (scaffolds), and growth factors. Engineering scaffolds provides three-dimensional supports for cell proliferation and growth of tissue-like structures, while growth factors play an important role in induction of bone formation and in maintaining the integrity of bone with proliferation and/or differentiation of osteogenic cells in vitro and in vivo [11,12].
Thus, the scaffolds play an important role in bone tissue engineering, and in general, required to have three-dimensional porous structure with high porosity, pore interconnectivity, uniform pore distribution, surface properties permitting cell adhesion, differentiation, non-cytotoxicity and osteoconductivity [13,14]. Porosity and interconnectivity are important for an accurate diffusion of nutrients and gases and for the removal of metabolic waste resulting from the activity of the cells proliferated into the scaffolds [15]. Therefore, the selection of the most appropriate materials to produce scaffolds is of importance because their original properties affect the potential properties of scaffolds greatly [16]. Bioceramics such as HA, - TCP and -TCP and biopolymers such as collagen, poly(jbgxdh gcct78-pdfty -caprolactone) (PCL) and poly(L-lactic acid) (PLLA) have widely been used in the scaffold development in bone tissue engineering. Those bioceramics have ideal properties as bone scaffolds such as bioactivity, biocompatibility and osteoconductivity [17-20]. On the other hand, those biopolymers are commonly used in tissue engineering fields in order to create more flexible structures [21,22].
Thus, HA-based porous materials and structures have been developed as bone grafts and scaffolds; however, their low mechanical properties such as modulus, strength and fracture absorbed energy have become problems and limited their wide applications in bone reconstruction and regeneration [23]. Furthermore, from the clinical point of view, the difficulty in handling due to the brittleness is also one of the main disadvantages in real surgical situations. To improve the extensive range of applications and use effectively in load bearing compartments, the mechanical properties of porous HA scaffold must be improved. One of the effective ways to improve such insufficient mechanical properties is to introduce the concept of composite material in which a polymer secondary phase is introduced into the HA materials [21,22,24-26]. However, there is a difficulty in combining HA and polymer because HA is usually sintered at very high temperature greater than 1000 ºC, at which most polymers are burned out easily.
In the present work, two different composite structures of porous HA and secondary PCL or PLLA phase were developed in order to improve the mechanical properties of a porous HA scaffold. In the first composite structure, PCL or PLLA membrane was coated on the surface of porous sintered HA scaffold. In the second composite structure, porous PCL or PLLA spongy phase was introduced into the continuous pores of the sintered HA scaffold. Their compressive mechanical properties were examined and compared and furthermore, effects of the polymer secondary phase on the compressive fracture micromechanism were also investigated.
Materials and specimen fabrication
Porous HA base scaffolds were fabricated by using the polyurethane (PU) sponge template method. HA slurry of 5wt% concentration was prepared from commercial micro-HA powder (PS-1, Sangi Co, Ltd.) containing particle size of 0.03 to 0.1 m mixed with poly vinyl alcohol (PVA: (CH2CHOH)n) (165-17915, Mw = 1500-1800g/mol, Wako Pure Chemical Industries, Ltd.) using a centrifuge mixing machine. PU sponge templates (HR-40, Bridgestone) cut into 1x1x1 cm3 were immersed into the slurry. The immersed sponge templates were then fully compressed to remove excess slurry, and dried at 60°C for 24 hours. Then, the PU templates with HA slurry process were preheated at 400°C for 6 hours in an electric oven and sintered at 1300°C for four hours.
PCL pallets with the glass transition temperature, Tg = -62.62°C and melting point Tm = 65.4°C (Celgreen PH7, Daicel Chemistry Industries Co., Ltd.) were dissolved in 1,4-dioxane to obtain solution with concentration of 1, 3, 5, 7 and 10wt% by stirring at 60°C for 2 hours. HA base scaffolds were then dipped into the solution and vacuumed for 1 hour to remove air. Two different conditions were employed in the drying process. In the first drying process, the soaked HA porous materials were taken out of the PCL solution and then dried in an air oven at a room temperature for 24 hours. These samples were named as ‘HA/PCL coated scaffold’. In the second drying process, the freeze-drying method was utilized to obtain unique two-phase porous structure in which PCL spongy phase is created inside the pores of the HA scaffold. The soaked HA base scaffolds were frozen at -20°C for 24 hours and then freeze-dried at -50°C for 24 hours. These specimens were named as ‘HA/PCL 2-phase scaffold’. PLLA pellets with the glass transition temperature Tg = 68.28°C and melting point Tm = 173.03°C (U’z B-3, Toyota Motor Co., Ltd.) were also used to fabricate two different composite structures using the exactly same procedures with HA/PCL composite scaffolds. These specimens were named ‘HA/PLLA coated scaffold’ and ‘HA/PLLA 2-phase scaffold’, respectively.
Characterization of microstructure
Micro-porous structures of the composite scaffolds were characterized using a filed-emission scanning electron microscope (FE-SEM S-4100, Hitachi, Ltd.). The specimens were attached on Al holders using carbon tapes and electro-conductive Ag paste (dotite D-550, Fujikura Kasei Co, Ltd.). The specimens were then coated with a thin layer of Pt-Pd using an ion sputter (Hitachi E1030 Ion Sputter) prior to the observation.
X-ray powder diffraction (XRD) was performed to determine the phase stability of the composite scaffolds using a X-ray diffractometer (Rigaku RINT-TTR III). HA powder, the raw material, and the milled powder of HA/PCL and HA/PLLA composite scaffolds were scanned from 2 of 20° to 40° with a step of 0.02°, while the HA/PLLA reinforced specimens were scanned from 2 of 10° to 40°.
Porosity values, ϕ, of the scaffolds were determined based on the density values of the composite scaffold, ρporous, and the bulk (solid) composite material, ρcomposite, by using the following formula:
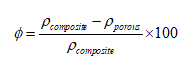 (1)
(1)
To determine ρcomposite, the Archimedes principle was applied. The weight change caused by the introduction of polymer secondary phase was also measured using an electronic balance. Increase in weight of composite scaffold, W, was determined in percentage using the following formula:
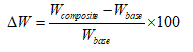 (2)
(2)
Where Wcomposite and Wbase are the weights of the composite scaffold and the HA base scaffolds, respectively.
Mechanical characterization
Compressive tests of the composite scaffolds were conducted to evaluate their mechanical properties by using a compact tabletop testing machine (EZTest, Shimadzu Co., Ltd.) equipped with a 500N load cell and a crosshead speed of 1mm/min. For each specimen, its dimensions, length, width and height, were measured prior to the test. Force and displacement were determined from the output of the load cell and the crosshead displacement, and then stress and strain were calculated from force and displacement data, respectively. Elastic modulus was estimated from the initial slope of the stress-strain curve. Compressive strength and fracture absorbed energy were also evaluated from the peak value of stress and the area of stress-strain curve up to the fracture point, respectively. For each condition, five samples were tested and the average value and standard deviation was evaluated.
Microstructural characteristics
Typical FE-SEM images of porous microstructures of HA/PCL and HA/PLLA coated scaffolds are shown in Figures 1(a) and (b), respectively. With 1wt% of polymer coating, both HA/PCL and HA/PLLA scaffolds basically maintained almost the same 3D porous microstructure consisting of highly interconnecting pores with pore size of about 100 m to 500 m. Such interconnection of pores is considered to be very important for cell proliferation and generation of tissue-like structures. In general, pore sizes larger than 100 m are thought to be preferable for tissue formation, transportation of nutrients and vascularization [14,27-33]. On the other hand, the pores were partially covered by 7wt% PCL and 3 and 7wt% PLLA. It is thought that increase in polymer concentration increased viscosity of the solution which resisted flowing out, induced some pores partially closed.
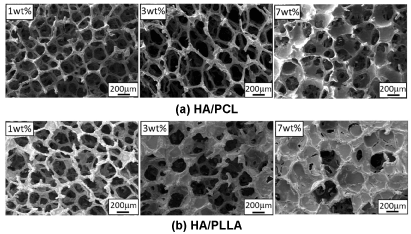
Figure 1. FE-SEM images of microstructure of HA/PCL and HA/PLLA coated scaffolds.
FE-SEM images of microstructures of HA/PCL and HA/PLLA 2-phase scaffolds are shown in Figure 2. The scaffolds showed three-dimensional porous structures with two types of pore arrangement, that is, pore arrangement created by the original HA porous structure and honeycomb-like polymer pore structure created by polymer solution under the freeze drying process. The polymer porous structure tended to become smaller in size due to increase in polymer concentration. It is also worth noting that PLLA pores became denser and smaller than PCL pores at the same polymer concentration.
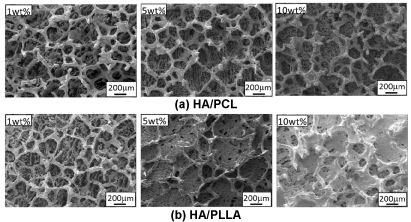
Figure 2. FE-SEM images of microstructure of HA/PCL and HA/PLLA 2-phase scaffolds.
Results of porosity and weight change measurements of both type of scaffolds are shown as a function of polymer concentration in Figure 3. HA/PCL coated scaffold exhibited decreasing behavior in porosity from 95 % to 89 % with increase of PCL concentration, while the porosity of HA/PCL coated scaffold decreased down to 87% at 10wt% PLLA. In contrast, the weight change of HA/PLLA coated scaffolds reached 80% with increasing PLLA concentration which was slightly higher than 74.6% of HA/PCL coated scaffolds. The porosity of the 2-phase scaffolds tended to decrease down to about 86% with increasing polymer concentration as shown in Figure 3(b). The weight change of the 2-phase scaffolds tended to increase linearly with increasing polymer concentration. At 10wt% of polymer concentration, the weight of HA/PLLA gained about 86% which is higher than 75.5% of HA/PCL.
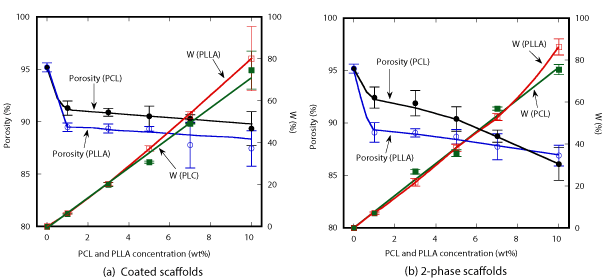
Figure 3. Porosity and weight change of HA/PCL and HA/PLLA scaffolds.
XRD patterns of the four types of composite scaffolds are shown in Figure 4. In the composite scaffolds, only typical PCL, PLLA and HA peaks were observed. There were no other peaks nor peak shifts shown in those composite scaffolds, suggesting that no chemical reaction occurred.
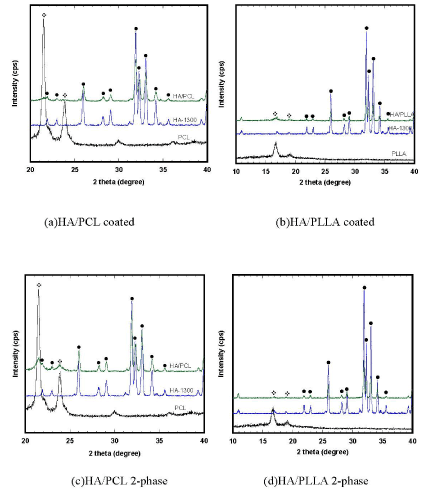
Figure 4.X-Ray diffraction patterns of pure PCL and PLLA polymers, HA base scaffold, and composite scaffolds (•) HA and (*) PCL/PLLA.
Compressive mechanical properties
Variations of elastic moduli and compressive strengths of the coated scaffolds are shown in Figure 5(a). Elastic modulus of HA/PCL coated scaffold tended to increase with increasing PCL concentration, starting from the initial value of 0.5 MPa of HA base scaffold up to the maximum value of 2.3 MPa. A linear increase in elastic modulus was observed up to 5wt% PCL, and then it remained steady. Elastic modulus of HA/PLLA coated scaffold increased up to 3.6 MPa with increasing PLLA concentration. A sharp increase was observed up to 3wt% PLLA, and then it continued to increase slowly. On the contrary, compressive strength of HA/PLCL coated scaffold significantly increased from 18 to about 107 kPa with increasing PCL concentration, while that of HA/PLLA rapidly rose up to 524 kPa. It is thus worth noting that HA/PLLA showed much higher values of both mechanical properties than HA/PCL, mainly due to the higher properties of PLLA.
Variation of elastic modulus and compressive strength of the 2-phase scaffolds are shown in Figure 5(b). Elastic modulus of HA/PCL 2-phase scaffold slowly increased up to about 1.6 MPa with increasing PCL concentration, while that of HA/PLLA increased almost linearly up to about 7.8 MPa with increasing PLLA concentration. The greater elastic modulus generated by the PLLA secondary phase was caused by the lower ductility of PLLA than ductile PCL with much lower glass transition temperature [34-39]. With the PCL secondary phase, the compressive strength effectively increased up to about 200 kPa at 10 wt% PCL, while with the PLLA secondary phase, the strength more significantly increased up to about 887 kPa. It was thus clearly shown that introduction of spongy polymer phase effectively improved the compressive mechanical properties.
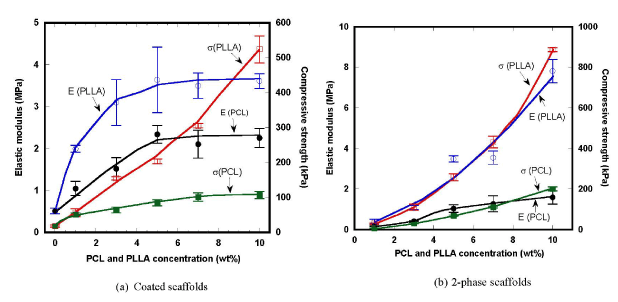
Figure 5. Variation of elastic modulus and compressive strength of the composite scaffolds.
Variational behaviors of fracture energy absorption of the composite scaffolds are shown in Figure 6. Both introductions of PCL and PLLA secondary phase are effectively improved the fracture energy due to the effects of energy dissipation by ductile deformation of polymeric phases. It was clary seen that the effects of PLLA were much more significant than those of PCL, mainly owing to the greater mechanical properties of PLLA. It is well known that PLLA creates farm spherical structures of crystals which result in the favorable mechanical properties comparable with engineering polymers.
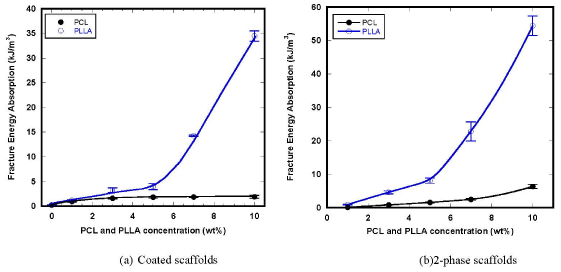
Figure 6. Variation of fracture energy absorption of the composite scaffolds.
It is worth noting that the compressive mechanical properties such as elastic modulus, strength and fracture absorbed energy of the 2-phase scaffolds were greater than those of the coated scaffolds. Thus, introduction of spongy polymer phase into the HA frame structure has better influence on the mechanical properties than polymer coating on the surface of HA struts. However, it is expected that the volumes of continuous pores of the coated scaffolds are much larger than those of the 2-phase scaffolds and hence, the coated scaffolds keep much more space for cell proliferation and construction of tissue-like structure inside of the pores.
Fracture mechanisms
Typical fracture micromechanisms of the coated scaffolds are shown with the stress-strain curves in Figure 7. The fracture behavior and the stress-strain curve of the HA base scaffold is also shown in these figures. The stress-strain curve of the HA base material showed an abrupt decrease in stress after the maximum point of stress due to typical brittle fracture behavior of porous ceramic structure. On the contrary, the coated scaffolds exhibited a decreasing behavior of stress after the maximum point mainly owing to ductile deformation of the polymer membrane supporting the gradual densification of the structure, resulting in dramatic improvement of fracture resistance.
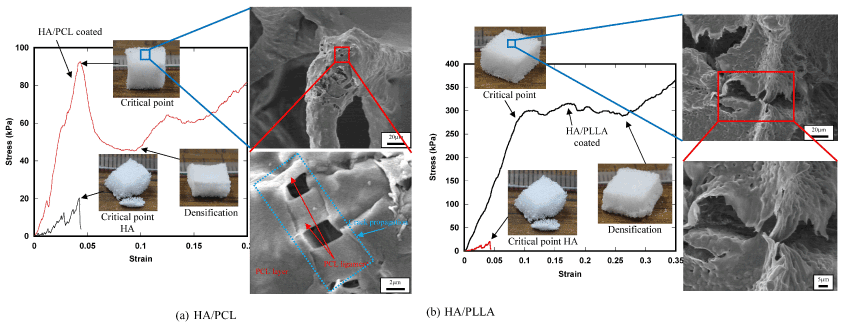
Figure 7. Fracture micromechanisms of the coated scaffolds.
Fracture micromechanisms of the 2-phase scaffolds are shown with the stress-strain curve in Figure 8. In Phase 1, stress-strain relation is almost linear, corresponding to macroscopic linear elastic deformation. In Phase 2, localized fracture of the HA frame structure took place and the number of fracture points gradually increased, resulting in the decrease of slope of the stress-strain curve. In the HA base scaffold, such localized fracture initiated the catastrophic fracture of the whole frame structure as shown in Figure 7, however, in the 2-phase scaffolds, spongy polymer phase supported the applied load and therefore, the scaffold kept the compressed tetrahedral shape as shown in the figure. It is worth noting that the plastic deformation of the ligament structures of polymer on the surface of the HA struts also played an important role for fracture resistance. Due to the load bearing effect of spongy polymer phase, the scaffolds underwent densification with compressed deformed shape (Phase 3).
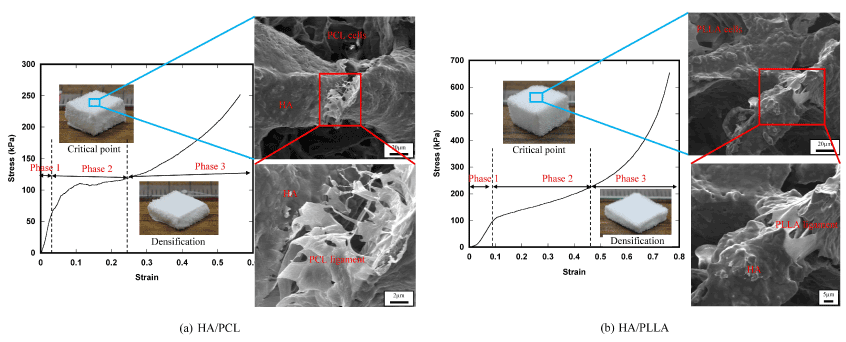
Figure 8. Fracture micromechanisms of the 2-phase scaffolds.
Those results of microscopy clearly indicated that the introduction of secondary polymer phase effectively worked as an energy dissipation mechanism during compressive deformation process, that is, mainly plastic deformation of the polymer phase.
In this study, novel porous composite scaffolds of bioceramic HA and bioabsorbable polymers, PCL and PLLA, were developed using two different reinforcing method to improve the fundamental compressive mechanical properties of pure HA base scaffold. The conclusions were obtained as follows:
- Introducing the new fabrication method combining the template method and the freeze-drying technique, we successfully developed a unique 2-phase porous structure in which HA created porous frame structure and polymer constructed honeycomb-like spongy structure.
- Both polymer coated and 2-phase scaffolds maintained three-dimensional continuous porous structures that are an ideal structure for cell proliferation, growth and construction of tissue-like structures inside of the pores.
- Compressive mechanical properties such as elastic modulus, strength and fracture absorbed energy were dramatically improved by introducing secondary polymer phase into the HA base scaffold. Especially, the 2-phase scaffold exhibited much better mechanical properties than the coated scaffolds. Furthermore, PLLA was more effective than PCL mainly owing to the greater mechanical properties than ductile PCL.
- The primary toughening mechanism appeared in the polymer composite scaffolds was thought to be the ductile deformation of the secondary polymer phase. Furthermore, the secondary spongy polymer phase created inside of the HA frame structure worked as a cushion that absorbed mechanical energy effectively and kept the entire porous structure preventing catastrophic fracture of the scaffolds.
This work was supported in part by the Collaborative Research Program of Research Institute for Applied Mechanics, Kyushu University.
- Damien CJ, Parsons JR (1991) Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater 2: 187-208. [Crossref]
- Salgado AJ, Coutinho OP, Reis RL (2004) Bone Tissue Engineering: State of the Art and Future Trends. Macromol Biosci 4: 743-765. [Crossref]
- Finkemeier CG (2002) Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am 84-84A: 454-64. [Crossref]
- Rose FRAJ, Oreffo ROC (2002) Bone tissue engineering: hope vs hype. Biochem Biophys Res Commun 292: 1-7. [Crossref]
- Spitzer RS, Perka C, Lindenhayn K, Zippel, H (2002) Matrix engineering for osteogenic differentiation of rabbit periosteal cells using a-tricalcium phosphate particles in a three-dimensional fibrin culture. Journal of Biomedical Materials Research 59: 690-696.
- Sutherland D, Bostrom M (2005) Grafts and bone graft substitutes. In: Lieberman JR, Friedlaender GE (Eds.), Bone regeneration and repair: Biology and clinical applications. Humana Press Inc.
- Schnettler R, Stahl J, Alt V, Pavlidis T, Dingeldein E, et al. (2004) Calcium Phosphate-Based Bone Substitutes. European Journal of Trauma 30: 219-229.
- Nather A, Aziz Z (2005) Allografts. In: Nather A (Ed.), Bone grafts and bone substitutes. World Scientific Publishing Co. Pte. Ltd.
- Ren LM, Arahira T, Todo M, Yoshikawa H, Myoui A (2012) A comparative biomechanical study of bone ingrowth in two porous hydroxyapatite bioceramics. Applied Surface Science 262: 81-88.
- Ren LM, Arahira T, Todo M, Yoshikawa H, Myoui A (2012) Biomechanical evaluation of porous bioactive ceramics after implantation: micro CT-based three-dimensional finite element analysis. J Mater Sci Mater Med 23: 463-472. [Crossref]
- Kneser U, Schaefer DJ, Munder B, Klemt C, Andree C, et al. (2002) Tissue engineering of bone. Minimally Invasive Therapy & Allied Technologies 11: 107-116.
- Sarvazyan N (2012) Creation of Living Tissue: An Engineering Feat. In: Obradovic B (Ed.), Cell and Tissue Engineering. Springer.
- Langer R, Vacanti JP (1993) Tissue engineering. Science 260: 920-926. [Crossref]
- Salgado AJ, Coutinho OP, Reis RL (2004) Bone Tissue Engineering: State of the Art and Future Trends. Macromol Biosci 4: 743-765. [Crossref]
- Freed LE, Vunjak-Novakovic G (1998) Culture of organized cell communities. Adv Drug Deliv Rev 33: 15-30. [Crossref]
- Gomes ME, Salgado A, Reis RL (2002) Bone Tissue Engineering Using Starch Based Scaffolds Obtained by Different Methods. In: Reis R, Cohn D (Eds.), Polymer Based Systems on Tissue Engineering, Replacement and Regeneration. Springer Netherlands.
- Hench LL (1991) Biorceramics: From concept to clinic. Journal of the American Ceramic Society 74: 1487-1510.
- Kitsugi T, Yamamuro T, Nakamura T, Oka M (1995) Transmission electron microscopy observations at the interface of bone and four types of calcium phosphate ceramics with different calcium/phosphorus molar ratios. Biomaterials 16: 1101-1107. [Crossref]
- Murugan R, Ramakrishna S (2006) Production of ultra-fine bioresorbable carbonated hydroxyapatite. Acta Biomater 2: 201-206. [Crossref]
- Tracy BM, Doremus RH (1984) Direct electron microscopy studies of the bone—hydroxylapatite interface. J Biomed Mater Res 18: 719-726. [Crossref]
- Azevedo MC, Reis RL, Claase MB, Grijpma DW, Feijen J (2003) Development and properties of polycaprolactone/hydroxyapatite composite biomaterials. J Mater Sci Mater Med 14: 103-107. [Crossref]
- Mourinño V, Boccaccini AR (2010) Bone tissue engineering therapeutics-controlled drug delivery in three dimensional scaffolds. J R Soc Interface 7: 209-227. [Crossref]
- Hulbert SF, Young FA, Mathews RS, Klawitter JJ, Talbert CD, et al. (1970) Potential of ceramic materials as permanently implantable skeletal prostheses. J Biomed Mater Res 4: 433-456. [Crossref]
- Guarino V, Causa F, Netti PA, Ciapetti G, Pagani S, et al. (2008) The role of hydroxyapatite as solid signal on performance of PCL porous scaffolds for bone tissue regeneration. J Biomed Mater Res B Appl Biomater 86: 548-557. [Crossref]
- Kim HW, Knowles JC, Kim HE (2004) Hydroxyapatite/poly(epsilon-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery. Biomaterials 25: 1279-1287. [Crossref]
- Park S, Lee S, Kim W (2011) Fabrication of porous polycaprolactone/ hydroxyapatite (PCL/HA) blend scaffolds using a 3D plotting system for bone tissue engineering. Bioprocess Biosyst Eng 34: 505-513. [Crossref]
- Hutmacher DW (2000) Scaffolds in tissue engineering bone and cartilage. Biomaterials 21: 2529-2543. [Crossref]
- Hutmacher DW, Garcia AJ (2005) Scaffold-based bone engineering by using genetically modified cells. Gene 347: 1-10. [Crossref]
- Lee SH, Shin H (2007) Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev 59: 339-359. [Crossref]
- Levenberg S, Langer R (2004) Advances in Tissue Engineering. Current Topics in Developmental Biology. Academic Press.
- Mikos AG, Temenoff JS (2000) Formation of highly porous biodegradable scaffolds for tissue engineering. Electronic Journal of Biotechnology 3: 1-6.
- Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR (2006) Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 27: 3413-3431. [Crossref]
- Shor L, Güçeri S, Wen X, Gandhi M, Sun W (2007) Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions in vitro. Biomaterials 28: 5291-5297. [Crossref]
- Todo M, Park SD, Takayama T, Arakawa K (2007) Fracture micromechanisms of bioabsorbable PLLA/PCL polymer blends. Engineering Fracture Mechanics 74: 1872-1883.
- Vieira AC, Vieira JC, Ferra JM, Magalhães FD, Guedes RM, et al. (2011) Mechanical study of PLA-PCL fibers during in vitro degradation. J Mech Behav Biomed Mater 4: 451-460. [Crossref]
- Tian T, Jiang D, Zhang J, Lin Q (2008) Fabrication of bioactive composite by developing PLLA onto the framework of sintered HA scaffold. Materials Science and Engineering: C 28: 51-56.
- Van De Velde K, Kiekens P (2002) Biopolymers: overview of several properties and consequences on their applications. Polymer Testing 21: 433-442.
- Zuideveld M, Gottschalk C, Kropfinger H, Thomann R, Rusu M, et al. (2006) Miscibility and properties of linear poly(l-lactide)/branched poly(l-lactide) copolyester blends. Polymer 47: 3740-3746.
- Kouya T, Tada SI, Minbu H, Nakajima Y, Horimizu M, et al. (2013) Microporous membranes of PLLA/PCL blends for periosteal tissue scaffold. Materials Letters 95: 103-106.

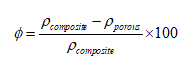 (1)
(1)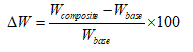 (2)
(2)






