Background: Young children are vulnerable to micronutrient (MN) deficiency especially in developing countries. This study aimed to determine the effect of nutrition behavior change intervention on improving micronutrients concentration and linear growth of children age 6 to 59 months in Central Highland of Ethiopia:
Design: A cluster-randomized controlled Community Trial was carried out from February 2017 to April 2019 for women who had children age 6 to 59 months. Comparing mean and Generalized Estimating Equations (GEE) models were fitted to children’s linear growth and identify predictors of child linear growth
Setting: The study was conducted in six districts found in the central highland of Ethiopia. Households (HH) and correspondent mothers/caregivers with their index children were included in the study assigned to the intervention group received monthly nutrition education and counseling.
Participants: About 1012 at baseline followed up and end-line data were collected for 815 study subjects.
Results: Iodine deference (ID) and Iron deficiency anemia (IDA) lowered to 6.15% and 8.83% respectively at the end of the intervention. The mean end-line-baseline difference (MD) of iodine concentration the differences between intervention and control groups (MD=198.38 μg/L P<0.05) and MD of hemoglobin concentration (Hbc) MD=3.87 g/L, P<0.05. On GEE analyses, Intervention group had increased height by 10.802 cm (β = 10.802, P<0.0001) and for one mg/dl increase in the baseline haemoglobin, end-line height increased by .086 cm (β =0.086, P=0.001) compared to other variables. Follow up studies are needed to determine the occurrence of IDD and related IDA health problems in the study area.
CI: Confidence interval; Diff: Difference; Hbc: Hemoglobin concentration; EDHS: Ethiopian demographic health survey; Ht: Height; GEE: Generalized estimating equations; HFA: Height for age; HH: Household; IDA: Iron deficiency anemia; ID: Iodine deficiency; IDD: Iodine deficiency disorder; IFPRI: The international food policy research institute; L: Litter; μg/L: Microgram per liter of urine; MD: Mean difference; MUIC: Median urine iodine concentration; NBC: Nutrition behavior change; Z: Z score, SD: Standard deviation; SE: Standard error; P: Significance; UIC: Urine iodine concentration; IC: Iodine concentration
The roles of micronutrients (MNs) during childhood have wide range purposes from the promotion of health to the curing of diseases, physical growth to mental development, and optimal for physiological function [1]. Children 6 to 59 months at the highest risk of MN deficiency, especially iodine and iron deficiencies increase the risk of morbidity and mortality [2,3]. These two micro minerals deficiencies have taken a prolonged time to limit the extent of health and growth-related problems [4]. Children's physical and intellectual deficits are potentially arising from iodine and iron [5]. These nutrients deficiencies are the most root cause of hidden hangar [6]. Also, the International Food Policy Research Institute (IFPRI) used stunting growth typically indicator of micronutrient deficiencies in affected populations and indicator of countries with high rank of Global Hidden hangar Index [GHI] [7]. Ethiopia is the tope country affected by iodine and iron deficiencies from the entire globe [8] and reference for the rising of high rank [70] of GHI [7].
The requirement of iodine during childhood is high in per kg of body weight which is needed to meet their rapid physical growth and mental development [9,10]. The daily requirement of iron is about 1 to 3 mg, but an intake of about 15 to 25 mg is needed due to poor absorption of iron [9].
The iron nutrient is the greatest challenge for preschool-age children's health [11] and currently near to 47.4% of these children affected by iron deficiency anemia [12]. Further, the risk of iodine deficiency (ID) among preschool children and their exposure to irreversible mental impairment and physical damage is still high [13]. The current UNICEF progress report indicates that more than 19 million infants were unprotected from the lifelong consequences of brain damage associated with iodine deficiency [6]. The development and growth of children significantly influenced by the nutrients that they received in the earliest years of life and this can make or break their chance of a prosperous future [14]. Poor and delayed access to iodized salt intervention for more than two decades in Ethiopia did not invite the initiation of measuring the inadequacy of iodine and its effect on the growth of young children [15], but among schoolchildren, great focus so far made [16].
In developing countries usually, MN individual subject supplementation trials have masked the community intervention implication [14]. However, sustainability community-based BCC intervention is an important approach to prevent micronutrient deficiency and improve the growth of children [17]. Additionally, community-based Nutrition Behavior Change (NBC) intervention enhances the community capacity to identify specific behaviors related to MN intake and helps encourage mothers/ caregivers to take change and self–monitor children’s MN intake through dietary diversification using locally available and affordable foods [18].
In Ethiopia, there was no data from community-based optimistic behaviors change to increase iron and iodine nutrient intake to promote the growth of children.
Study area
The study area was found in the central part of Ethiopia and characterized by a high range of rain and mountainous. This study area included the area near to the Arsi Bale plateau and the neighboring of Chilalo Mountain. Particularly, Tiyo, Limuna Bilalo, Digeluna Tijo from the rural districts, and Asella, Bkoji, and Sagure were from urban distracts included. From these highland districts, sixteen kebeles (lower administrative units) were selected and randomly assigned either to the intervention group or control group using Emergency Nutritional Assessment (ENA) software with consideration of a sufficient buffered zone.
Study period
Trial was carried out from February 2017 to April 2019.
Study design
Community-based Cluster Randomized Control Trial (CRCT) was employed to answer the study objectives.
Study sample size
The sample size was determined using Gpower 3.0 software assuming a power of 95%, precision of 5%, and an effect size of 0.25 giving 834. Additionally, for considering an increase in the age of children above 59 months old for the given intervention period was 11% =95. Where the design effect had 10% =83 and a final the estimated sample size was 1012.
Sampling method
Multistage sampling method was applied to select randomly assigned 16 kebeles equally eight into intervention and eight control clusters. Further, systematic random sampling used to select 506 households (HH) and correspondent mother-child couplets from randomly assigned intervention clusters kebeles known as the intervention group. At the same technique, 506 HH and correspondent mothers paired children selected from randomly assigned control kebeles.
During the first months of survey, assessment of mothers’ knowledge and attitude (KA) about prevention of IDD and iodized salt use and quantifying iodine found in table salt was done at household and wholesale market level [19]. A total of 812 and at end-line survey 715 children’s urine samples were collected and analyzed for median urine iodine concentration [UIC] [20,21].
Mothers/ caregivers were oriented about the need for requisition urine specimen collection from their children before distributing specimen collection clean cups. The demonstration was shown for mothers/ caregivers on how to keep urine specimens after collected from their children.
Measurements
The baseline urine samples collected in June 2018 and end-line samples were collected in April 2019. Consequently, collected urine samples were transported to Ethiopian Public Health Institute for analysis with ammonium persulfate for respective two survey periods, and the laboratory procedural steps used reported [20,21].
The cut-off value for iodine adequacy was indicated with the reference of WOH, MUIC 100 μg/L, and higher substantially adequate. However, MUIC blow 100 μg/L categorized as inadequate and this was also further categorized into insufficient [50 to 99 μg/L] and severe [<50 μg/L] iodine deficiency and MUIC used as an indicator of Ic in plasma thyroxin [22].
Hemoglobin (Hb) determination
Before blood samples collection, mothers/guardians were informed about the required blood sample and each child’s hand was warmed and relaxed for safe and precise finger prick at the child’s finger to prevent minimum risk. Trained laboratory technologists collected each child’s blood sample for baseline and endpoint surveys. Hemoglobin concentration (Hbc) was determined using HemoCue Hb 301® analyzer.
Each analyzed Hbc was adjusted for altitude and categorized into no anemia (Hb >110 g/L) as normal, anemic (Hb < 110 g/L) as anemia and clinical anemia was observed with Hb<70 g/L determined as sever Hb [23].
Anthropometric measurements
Children’s height and weight (Wt) were measured according to standard procedures recommended by the World Health Organization. Weight was measured using hanging spring balance for children age < 2 years and digital scale for children 2 years and above without shoes and with light cloths. Besides, height was measured barefoot using a stadiometer for children in the age group 24 months and above. For children less than 24 months, the recumbent length board was used for measuring the length and each child rested in a relaxed manner parallel to the long axis of the board, and measurement was taken using two trained data collectors. Weight reading and height reading was made to the nearest 0.1 kg and 0.1 cm, respectively. The average reading of two independent observers was used for the final analyses [24,25].
Data processing and analysis
Anthropometric and other data were entered into EpiData 3.0 software and transferred and analyzed using the Statistical Package for Social Science statistical software for Windows, version 21, Anthropometric data were exported to Emergency Nutrition Assessment (ENA) software to generate height for age Z-score (HAZ) and weight for height z-score (WHZ). HAZ ≥ -2 Z-score was categorized as normal growth status and HFA < −2 was labeled as stunted. Also, weight for height Z score (WHZ) was generated to determine wasting among children [24,25]. Anthropometric results of children's growth at baseline and end-line surveys were compared with the WHO 2005 growth standard using ENA software.
Mean end-line-baseline differences in micronutrient concentrations (iodine and iron) in the growth of children (Height and weight) were compared between intervention and control groups. The association between children's growth and different background characteristics of the study participants was done both for the control and intervention groups. The differences of difference between intervention and control groups and other variables were compared using T-test Pv <05. Variables that had P >0.2 on bivariate analyses were taken for further analyses with Generalized Estimating Equations (GEE). The results were presented using Beta coefficients (β) and 95% confidence intervals.
NBC interventional study had started within households (HH) of 1012 study participants and from this, 573 study subjects from intervention cluster and 439 from the control cluster. However, at the end-line survey, only 815 study subjects participated. From these, the intervention cluster contained 526 (64.5%) and control cluster contained 289(35.5%) children age 6 to 59 months, and their mothers/caregivers (Table 1).
Table 1. Children mothers’/caregivers’ socio- demographic status and their groups in Central Highland of Ethiopia, 2019
Variables |
Frequency (N=1012 baseline, n=812 endline) |
Percent |
Baseline |
|
|
Intervention group |
506 |
50 |
Control group |
506 |
50 |
Endline |
|
|
Intervention group |
506 |
62.09 |
Control group |
309 |
37.91 |
Maternal Age |
|
|
Age <20 |
119 |
14.6 |
Age 20-35 |
503 |
61.72 |
Age>35 |
193 |
23.68 |
Education Levels |
|
|
Illiterate |
186 |
22.82 |
Grade 1-4 |
166 |
20.44 |
Grade 5-8 |
273 |
33.62 |
Grade 9-12 |
190 |
23.4 |
Marital Status |
|
|
Single |
36 |
4.42 |
Married |
711 |
87.24 |
Divorced |
47 |
5.77 |
Widowed |
21 |
2.57 |
Occupation |
|
|
House wife |
466 |
57.17 |
Farmer |
244 |
29.94 |
Business women |
56 |
6.87 |
Gov/NGO employee |
7 |
0.86 |
Daily labourer |
42 |
5.16 |
Annul income (Birr) |
|
|
<1000 |
250 |
30.68 |
1000-5,000 |
446 |
54.72 |
5001-10,000 |
90 |
11.04 |
>10,000 |
29 |
3.56 |
n: endline sample; N: baseline sample
From a total of 1012 study subjects, 508 (50.20%) were <24 Months and this reduced to 237(29.08%) at the end line survey. However, children age >24 months were 504(49.80) and this increased to 578 (70.92%). The distribution of younger children age <24 and 174(73.42%) was included within intervention clustered and only 63(26.58%) were included in the control closeted group. Study subjects’ sex distribution resembled across the groups and during both baseline and end-line surveys (Table 2).
Table 2. Distribution of study subjects across intervention and control clustered groups during baseline and endline surveys, in Central Highland of Ethiopia
Variables |
Baseline assessment, n=1012 |
End line Assessment, n=815 |
|
Intervention (n [%]) |
Control (n [%]) |
Intervention (n [%]) |
Control (n [%]) |
Age |
|
|
|
|
> 24 Months |
217(42.89) |
287(56.72) |
332(65.61) |
246(79.62) |
< 24 Months |
289(57.11) |
219(43.28) |
174(34.39) |
63(26.58) |
Sex |
|
|
|
|
Male |
263(51.98) |
276(45.64) |
268 (52.96) |
148(47.90) |
Female |
243(48.02) |
230(40.80) |
238(47.04) |
161(52.10) |
HAZ |
|
|
|
|
> -2SD |
320(63.24) |
266(52.57) |
454 (89.72) |
156(50.49) |
< -2SD |
186(36.76) |
240(47.43) |
52(10.28) |
153(49.51) |
WHZ |
|
|
|
|
> -2SD |
439(86.76) |
464(91.70) |
470(92.89) |
180(58.25) |
< -2SD |
67(13.24) |
42(8.30) |
36(7.12) |
129(41.75) |
WAZ |
|
|
|
|
> -2SD |
424(83.80) |
424(83.80) |
473(93.48) |
141(45.63) |
< -2SD |
82(16.20) |
82(16.20) |
33(6.52) |
168(68.57) |
Hbc |
|
|
|
|
>110 g/L |
375(74.11) |
453(89.53) |
464(91.70) |
279(90.29) |
<110 g/L |
131(25.89) |
53(10.47) |
42(8.30) |
30(9.71) |
MUIC |
N=812 |
N=715 |
>100μg/L |
348(85.71) |
368(90.64) |
392(96.55) |
279(90.29) |
<100μg/L |
58(14.29) |
38(9.36) |
14(3.45) |
30(9.71) |
Hbc: Hemoglobin Concentration; HAZ: Height for Age Z-Score; MUIC: Median Urine Iodine Concentration; n: Number of study subjects; N: Urine samples for MUIC; WAZ: Weight for Age Z-Score; WHZ: Weight for Height Z-Score.
Subsequently, the baseline 812 and at end-line 715 study participants’ (children’s) urine samples were collected and analyzed. The median urine iodine concentration (UIC) among children during the endline survey was almost two times higher (209.9 μg/L, CI = 188.72, 231.0) than at the baseline survey (107.7 μg/L, CI=107.30, 108.34). The inadequacy of iodine (MUIC below 50 µg/l) among children during both baseline and end-pline surveys had not observed. Further, the adequacy iodine nutrient (MUIC >100) among children was 88.2% [n=716] (Table 2).
Iodine deficiency (ID) among study subjects was 11.8% (n=96) at baseline and reduced by half (6.15%) at the end-line. As result, iodide inadequacy was mild (MUIC = 50 to 99 μg/L), and severe form of ID (MUIC<50 μg/L) was not observed. From the entire ID, among the intervention group was 14.29% at baseline and reduced to 3.45%. Additionally, ID among the control group was 38 (9.36%) at baseline and showed a minor incline of 9.71% (Table 2).
The mean hemoglobin concentration among study subjects was higher (143 g/L+1.6) at the end-line survey compared to the baseline survey (125.5 g/L+ 1.73). The prevalence of IDA during baseline was mild (18.2%). The magnitude of IDA was higher (25.89%) among the intervention group during the baseline survey than the control group (10.47%). Nevertheless, at the end-line survey less proportion (8.83%) of IDA was observed and among the intervention group became lower by three times (8.30%) compared to the baseline finding (Table 2).
Regarding child growth, mean height 81.16 cm, ±13.6, and their mean weight was 11.42 kg±4.1. Along with these, at the end-line survey mean height and weight increased to 97.3 cm ±13.8 and 14.04±4.2 respectively.
Growth faltering/Stunting decreased from 36, 76% at baseline to 10.28% in the intervention group, while it increased from 42.10% at baseline to 49.31% at the end-line in controls. Similarly, the prevalence of wasting (weight for Height Z-Score [WHZ]) < - 2 decreased from 10.77% at baseline increased to 20.25% at the end-line. Among intervention group was 8.30%, while it increased in the controls from 8.30% at baseline to 41.75% at the end-line (Table 2).
As presented in Figure 1, the baseline distribution of height for age z-scores of children was shifted to the left for the who;e sample compared to WHO standard indicating the magnitude of stunting in the area. Conversely, the same figure weight for the distribution of height z-sore for the whole sample shows the presence of a double burden of malnutrition (Figure 1).
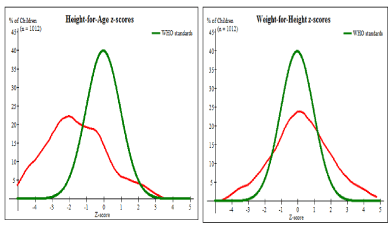
Figure 1. Growth of children age 6 to 59 months at baseline survey, 2017
Further, the prevalence of wasting growth (WHZ) among the intervention group was higher (13.24%,) at baseline compared to the control group (8.30%). Although at the end-line survey, wasting decreased by half (7.12%) among the intervention group and increased by five times (41.75%) among the control group. Weight for age (WAZ) among the study subject was 16.20% and decreased to 12.88% (Table 2).
At the end-line survey, the prevalence of stunting among study subjects reduced to the medium proportion (25.15%) and with larger SD: 1.783 but it should be between 1.10 - 1.30 SD and very far from WHO Anthro standard (Figure 2).

Figure 2. Growth of children age 6 to 59 months at end-line survey, 2019
Mean endline-baseline growth (Ht) difference of the differences between intervention and control groups was significant in (MD=0.51 cm, SE=0.98, pv=0.021) and in Wt (MD=1.292 kg, SE= 0.118, pv=0.0001). From socio demographic variables, the mean endline- baseline Ht and Wt difference of the differences between male and female children was insignificant in Ht (MD= -0.5 cm, SE=0.9) and in Wt (MD= -0.1 kg, SE=0.12). Also, no irrelevant differences between children age >24 months and <24 months in mean Ht (MD=0.59 cm, Se=0.35) and wt (Md=0.39 kg, Se=0.518). However, mean endline- baseline Ht and Wt difference among children who were introduced complementary food at 6 month differences among after 6 months were significant in Ht (MD=2.83 cm, Se=0.88, PV=0.013) and in Wt (MD=1.65 kg, Se=0.78, pv=0.027). Additionally, children from household with high monthly income had positive Ht difference than the less [MD=2.28, Se=0.83, p=0.01] (Table 3).
Table 3. Socio-demographic determined factors associated to Endline-baseline growth difference among children age 6 to 59 months in Central Highland of Ethiopia, 2019
Variables |
n=815(%) |
Mean |
Mean |
SD |
P |
Mean Wt |
M Wt Diff |
SD |
P |
Ht |
Ht Diff |
Groups |
|
|
|
|
|
|
|
|
|
Intervention |
526(64.54) |
11.94 |
|
4.3 |
|
2.65 |
|
3.92 |
|
Control |
289(35.46) |
10.45 |
0.51 |
10.9 |
0.02* |
1.36 |
1.29 |
0.88 |
0.0001* |
Sex |
|
|
|
|
|
|
|
|
|
Male |
416(51.04) |
10.87 |
|
11.7 |
|
2.14 |
|
4.63 |
|
Female |
399(48.96) |
11.37 |
-0.5 |
5.7 |
0.578 |
2.28 |
-0.1 |
1.21 |
0.244 |
Age Baseline |
|
|
|
|
|
|
|
|
|
> 24Months |
504(49.80) |
6.56 |
|
8.5 |
|
4.38 |
|
3.58 |
|
< 24Months |
508(50.20 |
5.97 |
0.59 |
3.5 |
0.358 |
3.99 |
0.39 |
0.58 |
0.187 |
Age endline |
|
|
|
|
|
|
|
|
|
> 24Months |
578(70.92) |
14.56 |
|
9.3 |
|
2.56 |
|
2.34 |
|
< 24Months |
237(29.08) |
13.87 |
0.69 |
5.1 |
0.458 |
2.01 |
0.55 |
0.16 |
0.185 |
Time compl. food |
|
|
|
|
|
|
|
|
|
6th month |
733(89.94) |
16.51 |
|
5.3 |
|
6.33 |
|
4.32 |
|
After 6th |
82(10.06) |
13.68 |
2.83 |
7.1 |
0.03* |
4.67 |
1.65 |
0.93 |
0.027* |
HH monthly inc. Et. Br |
|
|
|
|
|
|
|
|
|
>1000 |
454(55.70) |
11.01 |
|
3.8 |
|
8.07 |
|
5.7 |
|
<1000 |
361(44.29) |
8.73 |
2.28 |
6.3 |
0.01* |
7.46 |
2.6 |
2.64 |
0.258 |
Family Size |
|
|
|
|
|
|
|
|
|
4 to 6 |
662(81.23) |
5.49 |
|
2.6 |
|
6.11 |
|
3.67 |
|
7and > |
153(18.77) |
6.37 |
0.88 |
4.3 |
0.794 |
5.94 |
0.17 |
0.98 |
0.558 |
Br: Ethiopian currency; Diff: Differences; Et: Ethiopian; HH: Household; Ht: Height; n: Number of study subjects Wt: Weight; SE: Standard Error Difference; Pv: Significances
To major micronutrient concentration effect on children's growth in this study had a remarkable indication. The mean end-line-baseline difference of UIC in the intervention group was very high (204.567 μg/L) than control (29.286 μg/L). The mean end-line-baseline iodine concentration difference of the differences between intervention and control was significantly ascendancy (MD=175.28, SE=10.945, p=0.0001) and also with Hbc (MD=4.677, SE=0.686, p=0.0001).
There was mean endline-baseline UIC difference of the differences between standard (HAZ > -2SD) and stunted (HAZ < -2SD) groups was significant (MD = 35.094 μg/L, SE=11.43, p=0.002) extraordinary significant (MD=35.094 μg/L, SE=11.43, Pv=0.002) and WAZ (MD=60.916 μg/L, SE=18.31, pv=0.001).
The mean Hbc end line-baseline difference of the differences between intervention and control group was significant (MD= 4.677 g/L, SE=0.686, p=0.001). Further, mean end line-baseline iron concentrations (Hbc) difference of the differences in growth (WAZ) was positively considerable (MD= 2.05 g/L, SE=1,021, p-0.045) but, found no difference observed on HAZ (Table 4).
Table 4. Mean end line-baseline iodine, iron concentrations difference of the differences between groups and growth of children age 6 to 59 months, in Highland of Ethiopia, 2019
Variables |
Mean UIC (µg/l) |
Mean End line - baseline
MUIC difference (µg/l) |
SD |
P |
Intervention |
|
|
|
|
Yes |
204.5665 |
|
149.04 |
|
No |
29.2859 |
175.28 |
38.91 |
0.0001 |
Height for age Z score |
|
|
|
> -2 |
171.83 |
|
152.59 |
|
< -2 |
136.736 |
35.094 |
146.8 |
0.002* |
Weight for height Z score |
|
|
|
> -2 |
154.36 |
|
149.2 |
|
< -2 |
167.07 |
-12.72 |
156.97 |
0.353 |
Weight for age Z score |
|
|
|
> -2 |
163.889 |
|
|
|
< -2 |
102.973 |
60.916 |
18.31 |
0.001* |
Intervention |
|
|
|
Yes |
8.175 |
|
8.29 |
|
No |
3.498 |
4.677 |
7.59 |
0.0001* |
Height for age Z score |
|
|
|
> -2 |
6.532 |
|
8.29 |
|
< -2 |
7.479 |
-0.947 |
7.8 |
0.138 |
Weight for height Z score |
|
|
|
> -2 |
6.935 |
|
8.93 |
|
< -2 |
6.898 |
0.372 |
6.05 |
0.961 |
Weight for age Z score |
|
|
|
> -2 |
7.134 |
|
8.23 |
|
< -2 |
5.081 |
2.05 |
5.97 |
0.045* |
Hbc: Hemoglobin concentration; MD: Mean Differences; UIC: Urine Iodine Concentration; SE: Std. Error Differe`nce; Pv: Significances.
On GEE analyses, the intervention group had increased Ht by 10.802 cm (β = 10.802, P<0.0001) which showed that the followup through NBC intervention period had a significant effect with the growth of children. For one mg/dl increase in the baseline haemoglobin, end line-height increased by .086 cm (β = .086, P=0.001) compared to other variables. Also, those children who were following their growth monitoring in the health facility had higher end-line height by 2.214 cm (β =2.291, P= -0.027). Likewise, for a month increase in the age of the child, end-line height increased by .204 cm (B= .204, P<0.0001). For a unit increase in baseline HAZ, end-line height increased by .487 cm (B= .487, P=0.019). Similarly, for a month increase in the duration of breastfeeding, end-line height increased by .175 cm (P=0.003). Conversely, for a unit increase in baseline WAHZ, end-line height decreased by .620 cm (B=.175, -.620, P=0.019). However, both factors median urine iodine concentration (UIC) and iodine deficiency had not shown their impact on the growth of children (Table 5).
Table 5. Multivariable generalized estimating equations endline height of children age 6 to 59 months, in Central Highland of Ethiopia
Model |
|
|
95% CI |
P |
B |
Std. Error |
Lower |
Upper |
|
Intervention |
10.802 |
1.0225 |
8.798 |
12.806 |
<.0001 |
Sex=female |
-.687 |
.8908 |
-2.433 |
1.059 |
.441 |
Growth Monitoring |
2.291 |
.9560 |
.417 |
4.165 |
.017 |
Bottle feeding |
-.020 |
1.0013 |
-1.983 |
1.942 |
.984 |
Baseline HBC |
.086 |
.0257 |
.035 |
.136 |
.001 |
Baseline MUIC |
-.066 |
.0554 |
-.175 |
.043 |
.235 |
Baseline HFAZ |
.487 |
.2085 |
.078 |
.896 |
.019 |
Baseline WFHZ |
-.620 |
.2647 |
-1.138 |
-.101 |
.019 |
Household income |
.000 |
.0002 |
-7.449 |
.001 |
.113 |
Duration of breast feeding (Months) |
.175 |
.0599 |
.058 |
.293 |
.003 |
Education Level |
.101 |
.1312 |
-.156 |
.358 |
.442 |
Age(months) |
.204 |
.0365 |
.133 |
.276 |
<.0001 |
BF: Breastfeeding; CI: confident interval; Comp. food int.: Complementary food initiation; IDA: Iron deficiency anemia; Hbc: Hemoglobin Concentration; HAZ: Height for Age Z-Score; MUIC: Median Urine Iodine Concentration; UIC>100: Iodine suficency; WAZ: Weight for Age Z-Score; WHZ: weight for Height Z-Score.
We found out that at the end of the follow the intervention group was taller by 10.802 cm net of other factors adjusted in the generalized estimating equations model indicating the fact that the NBC intervention was effective. Other studies elsewhere have also shown a similar result [26,27]. This is an important finding implying the fact that such interventions at scale level could help to abate the high level of stunting that has been pervasively prevailing in Ethiopia [28]. In most African countries, the prevalence of stunting among underfive children varied from very high to medium levels. WHO/ UNICEF/World Bank reported that Burkina Faso was one of the countries that had medium proportion (26.8% in 2016 and 21.1% in 2017) stunted growth that invariable with the finding of this study at the end-line survey [29]. According to WHO < 20% stunting among children is low which 10.28% was after our micronutrient NBC intervention was provided for the intervention group, which is comparable to an interventional study conducted in the Amhara region that reported that stunting was lowered among intervention group than the control [30]. This could be possible with multifactorial micronutrient NBC interventions that ensure up to 70% nutritional and health status of people [31]. The mean end-line-baseline difference between the differences in height and weight were 2.83 cm and 1.65 kg, respectively. This could be possible within children who followed ordinary growth monitoring in the health facility and not challenging for BCC interventions. This also practically seen in our interventional study, that children who were regularly following their growth monitoring had a grater end line by over 2 centimeters than those had irregular/ no follow up. The possible reason for this could be that children who were attending growth monitoring could benefit from the timely counseling of maternal. Caregivers’ feeding and caring practices could enable them to grow normally, which s not the case ion those who were not following.
MUIC at baseline indicated that the status of adequate iodine nutrient among children was 88.2% and the iodide inadequacy was mild deficient (11.8%), although at end-line survey iodide inadequacy decreased to 6.15% and no severe form of iodide deficiency observed during both baseline and end-line surveys. This could be because of 99.17% of households were consuming iodized salt that contains sufficient amounts of iodine that is satisfactory to meet the requirement of iodine per person per day. Therefore, this study finding met the indication of WHO that referred to iodine deficiency not public health problem [29]. The mean endline-baseline MUIC difference of differences between intervention and control group was highly significant and differences observed among > HAZ (MD=35.094 μg/L) and >WAZ (MD= 60.916 μg/L) impressive. This could have happened with multiple integrated evaluations, monitoring, and educating the community about iodized salt utilization. This study was designed after a primary survey of iodized salt use in the household and quantification of iodine found in table salt reported that 99.1% of the households had met their requirement of iodine per person per day with mean 45.29+14.47 mg iodide /kg table salt [14]. As a result of introducing intensified NBC intervention on micronutrient for children mothers/ caregivers, a sufficient period promoted adequate intake of iodide through daily consumption of food. This could have contributed to the reduction in iodine deficiency in the intervention group. The findings imply that the need for intensifying NBC on iodized salt utilization at household level improve iodide status of children.
Mean iron concentration was significantly increased during the end-line survey than baseline. Also, the prevalence of anemia was 18.20% at baseline [31] which was considerably reduced to 8.83% at the end-line. In this study, the prevalence of anemia was higher (25.89%) among the intervention group at baseline was reduced to 8.30% at the end line, while among the control group had slightly increased from 9.71% to 10.47% at the end line. The prevalence of IDA documented by this study was mild which had no public health problem compared to WHO reference [32]. WHO /UNICEF recognizes anemia has been remained an unacceptable public health problem for many years with high prevalence in the globe [33,34]. This would be possible to reduce the prevalence of IDA through addressed dietary NBC on iron intake through the help of public health professionals and health extension workers. Behavioral change communications approach on micronutrient intake using dietary diversification and enhancement is used as part of food-based strategies for long-term success and sustainability of interventions to address nutritional anemia [35]. In this study for one mg.dl change in Hbc the end-line height of children increased by .086 cm. This finding had no similarity with the interventional study conducted in Burkina Faso reported that mean Hbc difference- found no significant impacts on children's growth [36].
The findings highlighted a significant positive impact of BCC on linear growth, iron, and iodine status of children. Hemoglobin concentration was significantly associated with the linear growth of children compared to iodine concentration. Therefore, urgent alternative and collaborative intervention required to reduce the burden of children's troubled growth.
Community based dietary BCC intervention is highly feasible and effective in the real-life context and needed to be considered for improving the nutritional and health challenges of children.
Acknowledgments
The authors express their gratitude to Jimma University for financial support. They extend their special thanks to Arsi Zone, health department, districts, and kebeles administrative officers and all health Extension Workers for their assistance and cooperation at the time of data collection and accompanying fieldwork and assisting our one year and three-month intervention program. Furthermore, a deep appreciation goes to Ethiopian Public health institute food and nutrition laboratory officers for their technical work in their laboratory.
Ethical approval
Ethical approval was obtained from the Ethical Review Committee of the Institutional Review Board (IRB) Institute of Health, Jimma University. A formal letter was written to the eight district Health offices and Health Extension Workers for permission and support until the intervention program completed. The study participants were informed that their participation was voluntary, and they were free to participate in the study, or refuse at any time and for any reason without any penalty. Informed consent for their paired children and their consent protocol was approved by the Ethical Review Committee.
Funding
The research was fully funded by Jimma University.
Competing interests
None declared.
Author contributions
FA did the design of the intervention project, analysis, and preparation of the whole manuscript, AM, participated in the analysis of data, write up and preparation and TB participated in the analysis of data, write up and final amendment of the manuscript for publication. All authors read and approved the final manuscript.
- Zimmermann MB (2012) The effects of iodine deficiency in pregnancy and infancy. Paediatric and Perinatal Epidemiology 26: 108-117.
- Black RE (2014) Global distribution and disease burden related to micronutrient deficiencies. Nestle Nutr Inst Workshop 78: 21-28.
- Kennedy G, Ballard T, Marie CD (2010) Guidelines for measuring household and individual dietary diversity; nutrition and consumer protection division, FAO. https://www.fao.org/3/i1983e/i1983e00.html. Accessed June 2016.
- IFPRI (2018) The international food policy research institute global food police report. https://www.ifpri.org/publication/2018-global-food-policy-report. Accessed June 2019.
- Allen, L. H. (2001) Biological mechanisms that might underlie iron’s effects on fetal growth and preterm birth. The Journal of Nutrition 131: S581-S589.
- UNICEF (2018) UNICEF progress report. https://www.unicef.Org/eca/press-releases/newborns-brain-damage-iodine-deficiency. Accessed December 2019.
- Bhutta ZA, Salam RA, Das JK (2013) Meeting the challenges of micronutrient malnutrition in the developing world. British Medical Bulletin 106: 7-17.
- IFPRI (2014) International food policy research institute global food policy report. Washington, D.C. https://www.ifpri.org/publication/global-nutrition-report-2014-actions-and-accountability-a. Accessed March 2016.
- United Nations FAO/WHO (2013) Expert consultation on human vitamin and mineral requirements. Food and agriculture organization of the united nations, 00100 Rome, Italy. http://whqlibdoc.who.nit/publications/2004/9241546123. Accessed January 2015.
- Zimmermann MB, Jooste PL, Pandav CS (2012) The effects of iodine deficiency in pregnancy and infancy. Paediatric and Perinatal Epidemiology 26: 1081117.
- Bailey RL, West Jr. KP, Black RE (2015) Epidemiology of global micronutrient deficiencies. Ann Nutr Metab 66: 22-33.
- WHO/CDC (2008) Worldwide prevalence of anaemia. 1993-2005: Global database on anaemia. Editors. Bruno de Benoist world health organization. Geneva, Switzerland. CDC. http://whqlibdoc.who.int/publications/2008/978924159665. Accessed January 2010.
- WHO, FAO, ICCIDD (2007) Iodine deficiency disorders and monitoring their elimination? A guide for program managers, third edition, World health organization. https://whqlibdoc.who.int/publications. Accessed April 2018.
- Allen L, Gillespie S (2001) What works? A review of the efficacy and effectiveness of nutrition interventions. https://www.ign.org/cm_data/what-works-nutrition-interventions_2001.pdf. Accessed March 2017.
- Pieter J, Maria A, Vincent A (2014) Iodine nutrition in Africa: The international council for control of iodine deficiency disorders (ICCIDD), global network. http://www.ign.org/cm_data. Accessed April, 2018.
- The iodine global network (2019) Global scorecard of iodine nutrition in 2019 in the general population based on school-age children IGN: Zurich, Switzerland. https://www.ign.orgcm_dataGlobal_Scorecard_2019_SAC.pdf. Accessed December 2019.
- Maher E, Zulliger K, Marcynyszyn L, Wilson D, Carroll CL, et al. (2013) Making the case for early childhood intervention in child welfare, a research and practice brief. https://caseyfamilypro-wpengine.netdna-ssl.com/media/EarlyChildhoodIntervention.pdf. Accessed June 2015.
- FAO (2011) Improving diets and nutrition food-based approaches. http://www.fao.org/3/a-i3030e.pdf. Accessed January 2016.
- Abebe F, Muluemebet A, Tefera B (2018) Knowledge and attitude about prevention of iodine deficiency disorders (IDD) among women and quantification of iodide found in table salt in central highland of Ethiopia. Biomedical Journal of Scientific & Technical Research 12: 9127-9135.
- Abebe F, Muluemebet A, Tefera B (2019) Median urinary iodine concentration and associated factors among children age 6 to 59 months in central highland of Ethiopia. Biomedical Journal of Scientific & Technical Research 17: 12938-12944.
- Abebe F, Muluemebet A, Tefera B (2020) Effect of behaviour change communication on iodised salt utilization and median urine iodine concentration among children growth age 6 -59 months in central highland of Ethiopia: Cluster randomized control trial. Biomedical Journal of Scientific & Technical Research 26: 20089-20097.
- WHO, UNICEF, ICCIDD (2007) Assessment of iodine deficiency disorders and monitoring their elimination? A guide for programme managers Third edition. Geneva 27, Switzerland. http://whqlibdoc.who.int/publications:2007/9789241595827. Accessed May 2013.
- World Health Organization (2011) Haemoglobin concentrations for the diagnosis of anemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization. WHO/NMH/NHD/MNM/11.1. http://www.who.int/vmnis/indicators/haemoglobin. Accessed September 2015.
- World Health Organization (1995) Physical status: the use and interpretation of anthropometry: report of a WHO Expert Committee. https://www.who.int/iris/handle/10665/37003. Accessed March 2013.
- World Health Organization (1997) Global database on child growth and malnutrition. Program of Nutrition. WHO. https://www.who.int/nutgrowthdb/about/introduction/en/index5.html. Accessed March 2015.
- Roy SK, Jolly SP, Shafique S, Fuchs GJ, Mahmud Z, et al. (2007) Prevention of malnutrition among young children in rural Bangladesh by a food-health-care educational intervention: a randomized, controlled trial. Food and Nutrition Bulletin 28: 375-383.
- Central Statistical Agency Ethiopia (2015) Ethiopia Demographic and Health Survey. https://dhsprogram.com/pubs/pdf/FR328/FR328. Accessed October 2017.
- Central Statistical Agency Ethiopia (2015) Ethiopia Demographic and Health Survey. https://dhsprogram.com/pubs/pdf/FR328/FR328. Accessed October 2017.
- UNICEF, WHO, World Bank (2019) Group joint malnutrition estimates: Levels and trends in child malnutrition. https://www.unicef.org/media/60626/file/Joint-malnutrition-estimates. Accessed January 2020.
- Karim B, Grace M, Frances A (2015) iodized salt improves child's iodine status, mental development, and physical growth in a cluster randomized trial in Ethiopia. The FASEB Journal 29: 1.
- WHO, UNICEF, ICCIDD (2001) Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers, 2nd edition, Geneva, USA. http://whqlibdoc.who.int/publications/2007/9789241595827_eng. Accessed October 2016.
- Ferede A, Abera M and Belachew T (2020) Haemoglobin level and associated factors among children age 6 to 59 months in central highland of Ethiopia, Int J Nutr Sci 5: 01-06.
- WHO/UNICEF/UNU (2001) Iron deficiency anaemia: assessment, prevention, and control. Geneva, World Health Organization. http://www.who.int/nut/documents/ida_assessment_prevention_control. Accessed July 2015.
- World Health Organization (2008) Indicators for assessing infant and young child feeding practices Part 1 Geneva, Switzerland. https://www.who.int/maternal_child_adolescent/documents/9789241596664/en/. Accessed September 2016.
- Badha J, Zimmermann MB, Kraemer K (2007) The guide book nutritional anemia, sight and life press. ISBN 3-906412-35-0.
- Deann KO, Abdoulaye P, Marie TR, Andrew D (2015) A 2-year integrated agriculture and nutrition and health behavior change communication, program targeted to women in Burkina Faso reduces anemia, wasting, and diarrhea in children 3-12.9 months of age at baseline: A cluster-randomized controlled trial. J Nutr 145: 1317-1324.

