A hybrid polymer drug delivery system (PEG-Chi) composed of linked poly (ethylene glycol) and chitosan chains was fabricated in handleable hydrogel and scaffold forms. The anticoagulant and highly anionic heparin drug model was loaded into the PEG-Chi matrix via scaffold incorporation and absorption methods. Application of an automated MATLAB-Arduino external pulsed electric current of 2 mA (at 5 cycles of 4 mHz for 20 min) led to the complete release of heparin molecules, while in a static condition (no current), 40% of the initial amount was retained. Heparin-electric current release was faster in absorbed versus the scaffold-integrated form. The negatively charged heparin, which was initially bound to the positively charged PEG-Chi matrix, was then electrostatically released into the medium through displacement by flowing electrons. Conversely, the cationic polylysine load was sequestered back into the matrix due to pulsed current after being released. This study demonstrated the regulated electrostatic movement of charged biologics into the hybrid PEG-Chi system that can ultimately be formulated for tissue engineering and localized clinical delivery of therapeutics.
pulsed electric current; drug delivery; electrostatic interaction; biomaterial scaffolds; poly(ethylene glycol); chitosan, heparin
Drug delivery systems (DDS) are vital to pharmaceutical and clinical sciences as they enable targeted therapeutics at controllable dosages within an effective timeframe [1,2]. They improve system efficiency by requiring smaller amounts of drugs that are otherwise cytotoxic at high concentrations, thus minimizing side effects. DDS can also be implanted in regions where the drug is needed to induce local action [3]. Controlled drug release can be obtained through stimuli-responsive or smart materials, such as intrinsically conducting polymers, which have organic molecular chains but possess charge conductivity like metals. These materials are currently being utilized for drug release with specific stimuli triggers, including magnetic fields, ultrasonic waves, and electric current [2,4]. Electrical stimulus may be the best trigger due to the relatively fast drug delivery rate [1] Electrostimulation studies have previously been conducted investigating drug release, modulated by pulsating current of low milliampere levels, from a polyelectrolyte hydrogel in vitro and in vivo (within mice) [5,6].
Most DDS are composed of hydrogels or networks of polymer chains that can sequester water molecules. Drugs can be incorporated into the gel matrix and subsequently released in an aqueous environment based on diffusion or stimuli. Hydrogel DDS materials include poly (propylene oxide), poly(lactic-co-glycolic acid), polypropylene fumarate, and poly(ethylene glycol) (PEG) [7]. PEG-based DDS are appealing due to their biocompatibility, mechanical tunability, and applicability to a variety of biomedical purposes [8]. PEG can be utilized as a resin for 3D printing of custom-shaped scaffolds to suit any geometric DDS requirements [9,10]. However, PEG gels alone are relatively weak (low tensile strength) and highly-porous which can lead to a very rapid drug release over a short period of time [11]. Another material of interest for DDS is chitosan (Chi), a copolymer of D-glucosamine (deacetylated component) and N-acetyl-D-glucosamine, due to its biocompatibility, degradability, hydrophilicity, and integration of amine (-NH2) groups in its matrix [12]. Multiple amine groups gain extra protons (-NH3+) in aqueous medium, thereby providing the chitosan matrix with net positive charges that can subsequently be used for electrostatic attraction of negatively charged drugs and therapeutics, including growth factors, antibodies, enzymes, and genes [13]. A combination of PEG and chitosan polymers may be an appropriate DDS material to address individual limitations.
Accordingly, in this study, a hybrid matrix (PEG-Chi) composed of PEG and chitosan was fabricated in both gel and scaffold (dehydrated gel) forms, and loaded with a model anionic drug, heparin. Heparin gains its highly negative charges from multiple sulfonic acid (–SO2OH) groups that release protons in water to become charged (–SO2O–). We investigated the release kinetics of heparin out of the PEG-Chi matrix over time at two different matrix formulations (gel versus scaffold), at two different heparin loading conditions (integrated during gel fabrication versus absorbed after scaffold synthesis), and at two different stimuli conditions (static or no electric current versus pulsed electric current applied). Additionally, a model cationic molecule containing multiple amine groups, polylysine, was integrated into the PEG-Chi matrix and its release behavior quantified after application of an external electric current.
Materials
Chitosan (ChitoClear®; 70% deacetylated from chitin) was obtained from Primex (Siglufjordur, Iceland), while Irgacure® 2959 (I2959) powder from BASF (Ludwigshafen, Germany). Common laboratory reagents, as well as, phosphate-buffered saline (PBS), poly (ethylene glycol) diacrylate (PEGDA; Mn = 700 g/mol), heparin sodium salt from porcine intestinal mucosa, and poly (L-lysine hydrobromide) (M = 3 × 104 to 7 × 104 g/mol) were purchased from Sigma-Aldrich (St. Louis, MO). Deionized water (with 18.2 MΩ⋅cm electrical resistivity) was used as the default solvent, unless stated otherwise. Electronic components: microcontroller, breadboard, resistor, and wires were acquired from Arduino (Somerville, MA), multimeter with ammeter function from Keysight Technologies (Santa Rosa, CA), and MATLAB software from MathWorks (Natick, MA).
Synthesis of PEG-chitosan (PEG-Chi) gels and scaffolds
Solutions of 2% (m/v) chitosan in 0.5 M acetic acid and 70% (m/v) I2959 in ethanol were prepared separately. Using these intermediate solutions and reagents, a master mix of 1% chitosan, 20% (v/v) PEGDA, and 1% I2959 were made then poured into 6-well plates at 5 mL per well. PEG polymerization [14] leading to solid gels was done by exposure to UV light at 254 nm for 7 min. Representative gels were fabricated into scaffolds by overnight freezing of gels at –80°C, then freeze-drying using FreeZone 2.5 Plus (Labconco, Kansas City, MO) for 3 days until complete removal of water. Gels and scaffolds were gently washed and rinsed thrice to remove unreacted components, and equilibrated overnight with PBS prior to use. These served as negative controls for the different experimental groups.
Mechanical characterization of gels via unconfined compression
Fabricated gels were characterized for their strength properties, specifically their ultimate compressive strength (UCS) and compressive modulus of elasticity (Ec) using the Instron 3345 mechanical tester (Norwood, MA) with a 5 kN load cell. Gels were biopsy punched into a cylinder with diameter of 5 mm and height of 5.5 mm, then placed on the sample platform. Unconfined compression testing was performed at a rate of 0.1 mm/s until failure as indicated by a sudden drop in force values. Data was normalized to zero at the start of load increase. The engineering compressive strain (εe) was obtained by dividing the displacement data by the initial height of the gel, while the engineering compressive stress (σe) was calculated by dividing the load data by the initial cross-sectional area of the gel. Engineering values were converted to true values using the formulas: true compressive strain ε = –ln(1 – εe) and true compressive stress σ = σe(1 – εe). The maximum σ was assigned as the UCS, and the slope of the initial linear elasticity region as the Ec.
Loading and release of drugs
Heparin (HEP), utilized as a negatively charged biologics drug model, was loaded into the PEG-Chi matrix at 17.5 mg (5 mL of 3.5 mg/mL) per sample of gel or scaffold via two methods: A) integration during gel fabrication and B) absorption after scaffold synthesis. In method A (integration loading), 35 mg/mL heparin solution was added to the gel master mix prior to photopolymerization to make a final concentration of 3.5 mg/mL. In method B (absorption loading), lyophilized scaffolds were washed and rinsed thrice with PBS, then soaked overnight in a 3.5 mg/mL heparin solution in PBS to a final volume of 5 mL. PEG-Chi only gels and scaffolds were used as “no drugs” controls.
Any residual medium was removed from the PEG-Chi-HEP samples and 5 mL of PBS was applied on top of the gel or scaffold samples. Two drug release methods were tested: 1) no electric current, and 2) with application of external pulsed electric current. In method 1 (diffusion release), 50 μL of PBS media were collected at the time points (tp): 0, 2, 6, 10, 14, and 18 min. In method 2 (electric current release), copper wire electrodes, separated by 30 mm distance, were placed into the PEG-Chi matrix at a depth of approximately 2 mm from the surface of the gel or scaffold and were soaked in the overlay PBS medium. The custom-made pulsed electric current system (Figure 1) was run at the settings: DC current = 2 mA, period = 4 min (equivalent to a frequency of about 4 mHz, with 2 min of constant 2 mA current and 2 min of no current); number of cycles = 5; and 50 μL of PBS obtained at tp: 0, 2, 6, 10, 14, and 18 min for analysis.
PLL was employed as a positively charged biologics drug model. PLL was incorporated into the PEG-Chi pre-hydrogel mix prior to photopolymerization at a final mass of 10 μg (5 mL of 2 μg/mL) per gel. After gelation and PBS washing, PLL was released out of the PEG-Chi matrix through diffusion and pulsatile electric current methods described above and 50 μL of samples were collected at tp: 0, 2, 6, 10, 14, and 18 minutes.
The collected drug release samples (drugs: HEP or PLL in PBS) and the corresponding control groups (PBS only) were analyzed for light absorbance (spectral scan) at 190 to 210 nm in a UV spectrophotometer. Standard curves (drug concentration versus absorbance) were obtained and fitted equations utilized to calculate the absolute concentrations of collected heparin and PLL in PBS samples. After subtracting the concentrations of blanks (PBS only), cumulative mass values were computed by multiplying with the PBS overlay volume, then release ratios (% released) obtained by dividing with the initial mass loaded into the gel or scaffold.
Pulsed electric current system setup
System design and configuration were based on the pulsed electric current generator developed in de Guzman laboratory [15]. The circuit diagram is illustrated in Figure 1A. A microcontroller pulse-width modulation (PWM) digital pin was assigned as an output and connected to the multimeter with ammeter functionality for DC current to verify the output electric current. An analog input signal was acquired to measure the voltage, and the average voltage (V) and the total resistance (R) were used to calculate the electric current (I) using Ohm’s law (I = V/R). Device control, signal acquisition, and analysis were performed using MATLAB. PWM output signals were calibrated to the circuit current based on ammeter data and computed values. Analog sensor voltage range was also obtained as a function of current by varying the resistances using different resistors. This enabled the conversion of analog voltages to electrical currents.
The resistance of the PEG-Chi matrix immersed in the PBS electrolyte at electrode separation distance (d) of 30 mm (Figure 1B) was computed by subtracting the total system resistance from the sum of the 220 Ω resistor and other circuit components resistance (13 Ω). The electrical conductivity (σ) [15] of the wetted PEG-Chi material was calculated from the equation: σ = I/(Vd).
To vary the electrical pulse at desired system (Figure 1C) settings (current = 2 mA, period = 4 min, and number of cycles = 5), a GUI program (Figure 1D) was written using MATLAB GUIDE (graphical user interface development environment) that enabled the control of pulse parameters.
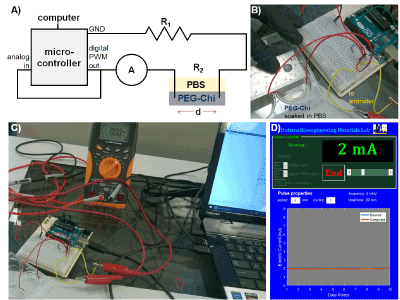
Figure 1: Pulsed electric current setup. A) Circuit diagram containing the following elements: microcontroller (with analog and digital pins and GND = ground), computer, A = ammeter, and R = resistance sources (R1 = resistor and R2 = PEG-Chi matrix with loaded drugs soaked in PBS). B) The electrodes in the PEG-Chi matrix were separated at a d = distance of 30 mm. C) Representative experimental assembly showing the actual devices. D) Screenshot of the graphical user interface (GUI) MATLAB program for controlling and displaying the electric current output of the system.
Sample management and statistical analyses
Experimental and control replicates were performed with sample sizes n ≥ 3. Calculated values and graphs were reported as average ± 1 standard deviation. Scatterplots, regression equations, and coefficient of determination (r2) fits were generated using MATLAB scripts. Student’s t-test, one-way analysis of variance (ANOVA) and post hoc multiple comparison tests were also analyzed in MATLAB with Statistics and Machine Learning Toolbox at 95% confidence intervals and 5% probability of type I error (α).
PEG-Chi gel mechanical properties
Control (PEG-Chi only) and experimental (with drugs) gels (Figure 2) and PBS-equilibrated scaffolds were opaque white in appearance, contributed by water-insoluble long chains of entangled PEG (20%) and chitosan (1%). Previous studies [11-13] have described the combination of PEG and chitosan as network chain materials. The hydrogels were firm, handleable, and retained water within their matrices. Smaller samples from biopsy punches were easily made which enabled the unconfined compression mechanical testing (Figure 3). It was determined that the compressive modulus (Ec) for fabricated PEG-Chi gels was 11.28 ± 2.40 MPa (n = 4), suggesting stiffer internal matrix structures than cartilages but softer as compared to bones [16]. Incorporation of the 1% chitosan into the PEG matrix increased the Ec by 3.6-fold (20% PEG gel only Ec = 3.10 ± 0.72 MPa). Lyophilization and rehydration (scaffold forms) significantly decreased (p < 0.001) the matrix Ec by 3.5 times [14]. The fracture or failure point, the ultimate compressive strength (UCS) property, was 9.41 ± 2.85 MPa. This value is similar to the lower spectrum of articular cartilage strength data [17], suggesting a potential biomechanical match for cartilage tissue engineering applications.

Figure 2: PEG-Chi gels. A) UV photopolymerization of a transparent pre-gel mix to form opaque gels. B) Appearance of gels loaded with heparin (HEP) and polylysine (PLL).
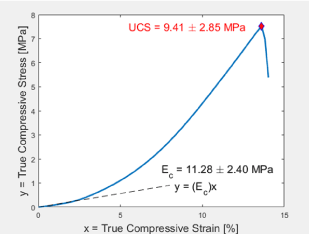
Figure 3: Representative stress-strain curve. Graph of true stress versus true strain of compressed PEG-Chi gel illustrating the initial linear elasticity region with the compressive modulus of elasticity (Ec) being the slope and the ultimate compressive strength (UCS) being the stress component of the fracture or gel failure point.
Instrument control and electrical behaviour
The electric current generation of the microcontroller assembly (Figure 1) was shown to be linearly (r2 = 0.999) dependent on the digital output PWM pin setting ranging from 0 to 1 (Figure 4A). The measured current through the ammeter and the calculated current matched per setting (minimal error = 1.71% to 2.08%). Additionally, the analog input voltage readings when used as an electric current sensor responded linearly (r2 = 0.997) to different current values; increasing current led to decreasing voltage measurements (Figure 4B). Using the derived regression equation: I = (5 – V)/0.022, where I = circuit electric current in mA and V = sensor voltage reading in V, the actual electric current was accurately quantified.
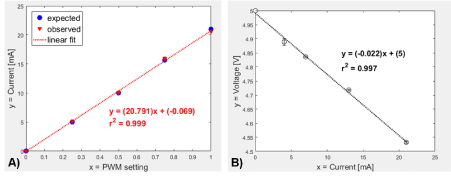
Figure 4: Microcontroller responses. A) Electric current ammeter output (expected) and computed microcontroller analog data (observed) responses versus the digital PWM setting output. B) Effect of analog voltage readings to varying electric current measurements using the custom setup. Linear regression model shown including the coefficient of determination (r2).
The calibrated device setup allowed us to determine the electrical properties of the PBS-equilibrated PEG-Chi matrix. At 2-mA flowing electric current, the total circuit resistance was found to be 1245 Ω. Accordingly, the PEG-Chi resistance was computed to be 1012 Ω and the associated electrical conductivity (σ) property equal to 27 mS/m. In comparison to the silicone-based matrix [15], the σ of PEG-Chi is about 5 times higher, suggesting greater ability to respond to flow of electrons.
Drug-release kinetics
Application of pulsed electric current (period = 4 min: 2 min on, then 2 min off) for 20 minutes using the MATLAB-Arduino controlled system (Figure 1) led to relatively higher release trend of heparin out of the PEG-Chi gels compared to those without electric current (Figure 5A), particularly at time points ≥ 6 minutes. In the absence of current, heparin release due to diffusion plateaued to about 60%, while gels exposed to 4 mHz of 2 mA electric current led to complete heparin release within 10 min of exposure (p < 0.05). The flow of electrons from the negative electrode into the gel matrix and PBS then to the positive electrode induced the displacement of matrix-bound heparin out into the PBS medium. The electrostatic attraction between the positively charged chitosan [18] network chains and the highly negatively charged heparin [19] enabled the initial sequestration of the heparin into the gel. Free electrons in the current have higher affinity (more negative charges) [13] to the chitosan, thereby releasing the ionically-held heparin (Figure 6). The fluctuating release kinetics of heparin appeared to be coincident with the 4-minute period of the applied external current. Delivery of low milliampere [15,20] pulsatile current may be more advantageous than continuous current for potential cell-based applications of this PEG-Chi matrix system.
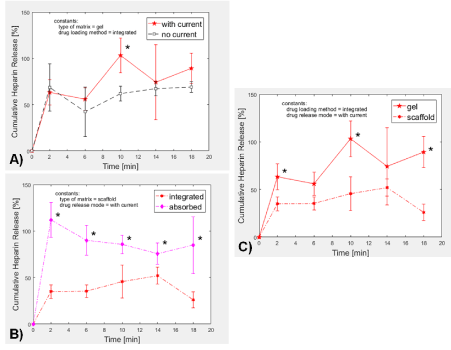
Figure 5: Heparin release from PEG-Chi. A) In gel formulations where heparin was loaded via integration, pulsed electric current induced relatively higher release ratios of heparin than diffusion-mediated (no current) samples. B) In scaffold forms (rehydrated lyophilized gels), external electric current application led to faster heparin release when heparin was initially loaded through absorption as compared to when heparin was integrated into the PEG-Chi during matrix polymerization. C) Matrix-integrated heparin release was generally higher in hydrogel matrix as compared to the release level in scaffold under pulsed electric current condition. *p < 0.05.
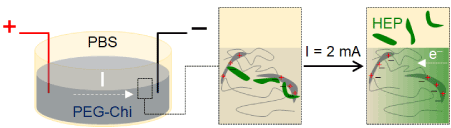
Figure 6: Mechanism of heparin release. PEG-Chi matrix-bound negatively-charged heparin (HEP) is displaced into the PBS overlay medium by flowing electrons (e–) due to application of an external electric current. Electrons electrostatically associate with the positively charged amine residues of chitosan.
Drugs in the bulk biomaterial matrix experience different release profiles depending on the nature of their loading process. Under pulsed electric current, heparin molecules that were absorbed into the PEG-Chi scaffold were released into the PBS overlay medium faster (p < 0.05) at all time points than when they were added prior to photopolymerization (Figure 5B). Complete release of absorbed heparin was observed just after 2 minutes, suggesting that anionic drug delivery is faster when drugs are absorbed into the scaffold.
When heparin was integrated into the PEG-Chi matrix and pulsed electric current was then applied, significantly higher (p < 0.05) release of heparin was found in those from gels as compared to those from scaffold forms at the 2, 10, and 18-min time points (Figure 5C). This indicates that the heparin affinity to the chitosan matrix increases during the process of freeze-drying, leading to stronger bulk interaction and slower release. Heparin was completely released out of the gels, but only 52% from scaffolds.
Gel-integrated positively charged PLL peptides were electrostatically repelled out of the positively charged PEG-Chi network (Figure 7). At the 2-min time point, 89% of the loaded drug model was released under the no current condition. Conversely, application of pulsatile electric current to the PBS-soaked gel seemed to sequester the free PLL back into the matrix since the PLL release ratio was down to 40%. There was a 29% to 49% range of difference between the releases of PLL at “no current” versus “with current”, implying that the negatively charged flowing electrons within the biomaterial matrix attracted the positively charged PLL.
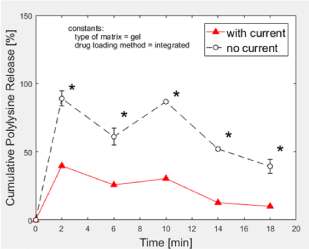
Figure 7: Polylysine release from PEG-Chi. Matrix-integrated polylysine was released at lower levels out of gels under pulsed electric current stimulation. *p < 0.05.
The study presents the fabrication of gels and scaffolds composed of an initial premix of 20% PEG and 1% chitosan through a photopolymerization reaction. These PEG-Chi constructs have cartilage-like strength and compressive modulus, and electrical conductivity value higher than silicone polymers. The anionic drug model, heparin, was loaded into the bulk matrix via integration prior to gelation, as well as through absorption. This study shows that the release kinetics of heparin is generally dependent on electrostatic interaction forces. Competing negative charges of flowing electrons lead to faster heparin release out of the positively charged network chains, while the release behavior of the positively charged polylysine follows an opposite pattern. Electric current application can induce the sequestration of free polylysine, hence, a slower release trend. Studies involving the stability, biocompatibility, responsiveness of PEG-Chi matrix materials to varying intensities and frequencies of electric currents, and in vivo drug release bioactivities will all help improve this promising biomaterial technology and move it forward towards clinical translatability.
The authors would like to thank the members of the Bioengineering Materials Lab including Emily Diaz, Jennifer Miller, Liam Lang, and Hazel Consunji for technical assistance, manuscript proofreading, and discussion, Jacqueline Scarola and Lori Castoria for help with reagents purchasing, and Sina Rabbany for his continuing support and collaboration in conducting biomaterials research at Hofstra SEAS. Support for this study was provided by Hofstra University internal research funds.
2021 Copyright OAT. All rights reserv
Compliance with ethical standards
Not applicable
Conflict of interest
The authors declare that there is no conflict of interest of a scientific or commercial nature. The authors have no relevant affiliations to, or financial support from any organization that may have a financial interest in the subject matter.
- Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, Bannerjee SK (2012) Drug delivery systems: An updated review. Int J Pharm Investig. 2: 2-11. [Crossref]
- Bazban-Shotorbani S, Hasani-Sadrabadi MM, Karkhaneh A, Serpooshan V, Jacob KI, Moshaverinia A, Mahmoudi M (2017) Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J Control Release. 253: 46-63. [Crossref]
- Jain V, Jain S, Mahajan SC (2015) Nanomedicines based drug delivery systems for anti-cancer targeting and treatment. Curr Drug Deliv. 12: 177-191. [Crossref]
- Svirskis D, Travas-Sejdic J, Rodgers A, Garg S (2010) Electrochemically controlled drug delivery based on intrinsically conducting polymers. J Control Release. 146: 6-15. [Crossref]
- Murdan S (2003) Electro-responsive drug delivery from hydrogels. J Control Release. 92: 1-17. [Crossref]
- Ge J, Neofytou E, Cahill TJ, 3rd, Beygui RE, Zare RN (2012) Drug release from electric-field-responsive nanoparticles. ACS Nano. 6: 227-233. [Crossref]
- Liechty WB, Kryscio DR, Slaughter BV, Peppas NA (2010) Polymers for drug delivery systems. Annu Rev Chem Biomol Eng. 1: 149-173. [Crossref]
- Alexander A, Ajazuddin, Khan J, Saraf S, Saraf S (2014) Polyethylene glycol (PEG)-Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Eur J Pharm Biopharm. 88: 575-585. [Crossref]
- Knowlton S, Yu CH, Ersoy F, Emadi S, Khademhosseini A, Tasoglu S (2016) 3D-printed microfluidic chips with patterned, cell-laden hydrogel constructs. Biofabrication. 8: 025019. [Crossref]
- Hong S, Sycks D, Chan HF, Lin S, Lopez GP, Guilak F, Leong KW, Zhao X (2015) 3D printing of highly stretchable and tough hydrogels into complex, cellularized structures. Adv Mater. 27: 4035-4040. [Crossref]
- Jung S, Yi H (2012) Fabrication of chitosan-poly(ethylene glycol) hybrid hydrogel microparticles via replica molding and its application toward facile conjugation of biomolecules. Langmuir. 28: 17061-17070. [Crossref]
- Chang FC, Tsao CT, Lin A, Zhang M, Levengood SL, Zhang M (2016) PEG-chitosan hydrogel with tunable stiffness for study of drug response of breast cancer cells. Polymers (Basel) 8. [Crossref]
- Ma G, Zhang X, Han J, Song G, Nie J (2009) Photo-polymeriable chitosan derivative prepared by Michael reaction of chitosan and polyethylene glycol diacrylate (PEGDA). Int J Biol Macromol. 45: 499-503. [Crossref]
- de Guzman RC, Rabbany SY (2016) PEG-immobilized keratin for protein drug sequestration and pH-mediated delivery. J Drug Deliv. 1-9. [Crossref]
- Jamal D, de Guzman RC (2017) Silicone substrate with collagen and carbon nanotubes exposed to pulsed current for MSC osteodifferentiation. Int J Biomater. 1-9. [Crossref]
- Butcher DT, Alliston T, Weaver VM (2009) A tense situation: forcing tumour progression. Nat Rev Cancer. 9: 108-122. [Crossref]
- Fung YC (1993) Biomechanics: mechanical properties of living tissues. New York: Springer-Verlag.
- Hamman JH (2010) Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar Drugs. 8: 1305-1322. [Crossref]
- Xiong S, Zhang L, He QY (2008) Fractionation of proteins by heparin chromatography. Methods Mol Biol. 424: 213-221. [Crossref]
- Hronik-Tupaj M, Rice WL, Cronin-Golomb M, Kaplan DL, Georgakoudi I (2011) Osteoblastic differentiation and stress response of human mesenchymal stem cells exposed to alternating current electric fields. Biomed Eng Online. 10: 9. [Crossref]







