The utilization of plant food for therapeutic purposes can be seen as the biggest regard for natural flora, which provides bio-active materials that have medicinal values. Dietary fibres are acknowledged to be of significance. What has yet to be articulated is the composition of fibre in plant foods. More specifically in this commentary, the fibre in edible processed cassava product and the potential to make fibre supplement from the otherwise waste products have yet to be appreciated. Indeed, cassava has yet to be included in several studies on antidiabetic plants. It is known that cassava may be high in substances that are regarded harmful to humans, but about 80% of it is removed during processing of the tuber. What this commentary brings to the fore is that soluble fibres in cassava include uronic acid, pectin and β-glucans. These have nutraceutical values including hypocholesterolemic and hypoglycemic effects needed in diabetes management. These can be extracted to produce supplement of naturally-occurring dietary fibre that lowers plasma LDL, VLDL-cholesterol and triglycerides and blood glucose. This potentially improves the agricultural economics and medical nutritional values of cassava.
carbohydrate food crop, diabetes management, fibre content, fibre supplement, health value
In the health sector, studies on nutritive and phytochemical composition of cassava have reported different medicinal values. Though, what is common in the various reports is that none mentioned use of cassava for diabetes and dyslipidaemia management [1]. While there is opinion that cassava could be a healthier choice than wheat and white potatoes for diabetes patients [2], there is no scientific evidence in the literature on the use of cassava for diabetes and dyslipidaemia management. Further, there is no arguing the fact that a high-fiber diet is therapeutic [3], but diabetes patients are being discouraged from consuming cassava in favour of wheat [4]. Indeed, there has been furor over cassava [5]. The problem arising from this is that accessible and affordable staple carbohydrate food crop, cassava, is losing value on health grounds in favour of imported foods products.
The furor calls for elucidation of information on international databases. For instance, a cursory review of the Australian National Survey Food Nutrient Database, comparing cassava with three other carbohydrate foods including wheat, will reveal that the energy with dietary fibre intake of wheat is 1119 kJ and without the fibre content is 784 kJ; whereas cassava is 587 kJ and 550 kJ respectively [6]. What is salient is that when the fibre content of the products are removed in processing, wheat loses most calorie than cassava. The information also indicate that wheat appears to have x9 of the fibre content of cassava (Table 1). However, a more critical review will show that while in the unprocessed food materials, low calorie/high fibre ratio is best in wheat compared to the others, low fat/fibre ratio is best in cassava (Figure 1).
Table 1. Some proximate nutrient values of cassava compare to other carbohydrate foods
Food ID |
Survey ID |
Food Name |
Energy+ ibre (kJ) |
Energy- fibre (kJ) |
Protein (g) |
Total fat (g) |
Starch (g) |
Total sugars (g) |
Dietary fibre (g) |
13A11691 |
24302009 |
Cassava* |
587 |
550 |
1.1 |
0.2 |
29.2 |
1.2 |
4.6 |
13A12015 |
24302043 |
Sweet potato* |
369 |
351 |
1 |
0.2 |
16.4 |
3 |
2.3 |
13A11740 |
24302049 |
Taro* |
469 |
441 |
1.9 |
0.2 |
22.3 |
1.1 |
3.5 |
02A10355 |
12101031 |
Wheat bran* |
1119 |
784 |
14.8 |
4.1 |
19.9 |
2.7 |
41.8 |
*Fresh, frozen, peeled, or raw
**unprocessed, uncooked
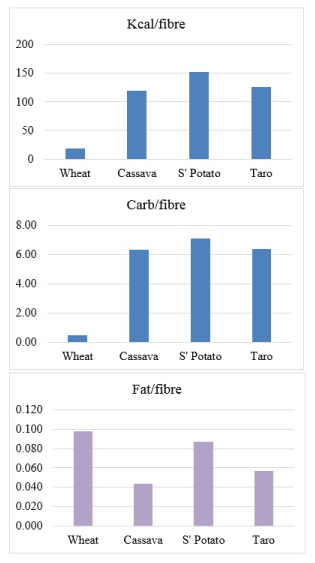
Figure 1. Comparison of nutrient-fibre ratios in individual products [6].
It should be interesting to note that in patients with diabetes and/or metabolic syndrome, weight reduction is desired and high fibre diet could help in this regard [7]. In previous review [8], it has been explained that there is inconsistent fat/fiber ratio inferences of ≤5 and ≤25 [9,10]. Further, it is common practice to mix wheat flours with grinded products of cassava and yam. Bearing this in mind, comparison of the mixture cassava, taro and wheat contributions to the calorie, carbohydrate, fat and fibre in edible content show that cassava contributes almost equal amount of calorie, highest amount of fibre and least amount of fat (Figure 2).
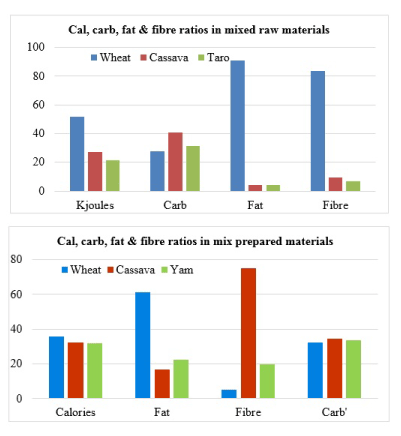
Figure 2. Comparison of macronutrient contributions of different items in food mixtures [2,6,65].
Focus on fibre as a therapeutic ingredient
Cassava may be high in substances that are regarded harmful to humans, but about 80% of it is removed during processing of the tuber [11]. Some traditional foods such as root/tuber crops have been found to be of great importance in the management of diabetes globally due to the presence of many biochemical [12]. The Food and Nutritional Research Institute has performed a short-term investigation on the glycemic index and cholesterol-lowering effect of root/tuber crops and discovered that they are low in glycemic index (GI ≤ 55), because they release their glucose gradually into the blood [13]. According to the result, root/tuber crops have potential health benefits in the prevention for risk of chronic diseases such as cardiovascular diseases and diabetes mellitus.
Diabetes mellitus is a metabolic disorder that affects the way the body handles basic food components like carbohydrates, protein and fats. In the last 40 years, many people have become interested in promoting the potentials of indigenous plant foods in developing countries and utilizing them into modern health system, this is as a result of high cost of Medicare and side effect of therapeutic drugs [14].
The utilization of plant food for therapeutic purposes can be seen as the biggest regard for natural flora, which provides bio-active materials that have medicinal values [15]. Flora has made significant contributions towards the treatment of ill-health. The reliability on the use of medicinal plants in the developed societies has been traced to the extraction and development of several drugs from plants [16,17]. While dietary fibres are acknowledged to be of significance, what has yet to be articulated is the composition of fibre in plant foods [18-21]. For instance, a dietary fibre comprises soluble and insoluble types. Yet, nutrient databases indicate mainly the total fibre content with delineation into the sub-types.
Soluble fibre: Root/tuber crops are good sources of dietary fiber with up to 14% content [13]. Dietary fiber has been shown to have important health implication in the prevention of risk of chronic diseases such as cardiovascular diseases and diabetes mellitus[13]. Fiber has the ability to bind with bile acids and prevent its reabsorption in the liver thus, inhibit cholesterol synthesis. The vicious and fibrous structure of dietary fiber can control the release of glucose with time in the blood, thus help in the proper control and management of diabetes mellitus and obesity[13].
Cassava contains 40% of soluble fiber, which consists mainly of uronic acid, pectin and β-glucans [22], whereas the insoluble fraction is rich in cellulose and lignin [23]. There has been increasing drive to include food rich in fiber in the daily diet, and for this purpose cassava could be utilized because of its high content in dietary fibre. In particular, dietary fibres depending carbohydrates form of food and starch structures, are important determinants of low glycemic index food [23-25].
In a research conducted on the glycemic index of commonly consumed carbohydrate foods in Philippines [26], they reported that root/tuber crops are low in GI. Reducing the glycemic impact of diet using foods low in glycemic index has been shown to improve overall blood glucose control in patients with diabetes mellitus [27].
Insoluble fibre: Currents reports suggests that resistant starch (RS) could be beneficial in preventing and managing metabolic syndrome by it process in delaying the rate at which glucose is delivered as fuel[28] . [29]Suggest that resistant starch is beneficial in the management of type 2 diabetes in human. Cassava has been reported to be very rich in resistant starch [30]. Resistant starch is defined as that fraction of starch, which escapes digestion in the small intestine and passes into the large intestine where it is more or less fermented by gut micro-flora. It is considered a functional component of food due to the health benefits it confers following its consumption. Resistant starch (RS) is naturally found in starchy foods such as cassava, potato, corn and rice.
Fact sheets of knowledge regarding dietary fibre in diabetes management: Some things are already known, which should form the basis of developing fibre supplements from roots and tuber crops as well as establishing the soluble fibre composition of staple carbohydrate foods.
- High-fiber diets, especially of the soluble fraction offer nutraceutical value in the improvement of carbohydrate metabolism, as it lowers cholesterol [18,19]. There is evidence of negative correlation between the amount of fibre content of food and the post-prandial blood sugar level [20]. Although some report appear to make this controversial [23], ensuring dietary fibre in meals diabetes patients is not only beneficial or encouraged [21], but recommended to be up to 14 g/1000 kcal of foods [25].
- Soluble fibres in cassava include uronic acid, pectin and β-glucans [22].
- Mushroom’s β-glucans has been acknowledged for its nutraceutical values including the hypocholesterolemic and hypoglycemic effects [31]
- Formulating dietary supplement for diabetes management requires multiple functional ingredient [32], which now includes fibre [33-35].
- A study has clearly stated that raw and boiled cassava tubers and leaves contain different bioactive compounds which may have different medicinal values [1], but made no mention on use of cassava for diabetes and dyslipidaemia management. Another study team reviewed antidiabetic and hypolipidaemic potentials of a their country’s flora [36], but never included cassava. Further, diabetes patients are advised to limit consumption of their staple carbohydrate foods [37]. At least, these reports constitute evidence that the soluble fibres in cassava are yet to be appreciated as a nutraceutical value in the staple food crop. Even in countries where root and tuber crop meals are the main staple, their actual food compositions have yet to be exhaustively documented in currently available databases [38].
As previously reported, there is absence of pharmacological data on the health economics’ value of cassava in diabetes and dyslipidaemia management [8]. The issue of interest is that chief among staple food crops, worldwide, is cassava [39,40]. However, several studies emphasize its toxic potentials [41,42], thereby overshadowing the medicinal values. Many studies reflect on the glycaemic index [2,5,43-45], without recourse to the impact of processing [46]. This overshadows the potential that cassava can lower cholesterol level in diabetes patients [47]. Yet, it is known that cassava contains alkaloids and flavonoid glycosides with medicinal values [48, 49], as well as fibre [2], which can be translated for medical nutrition therapy management of diabetes and its cardiovascular complications including heart disease [50].
Cassava as a global carbohydrate food crop: implications for international adoption
About 60% of the world production of cassava is concentrated in five countries that spreads across Africa, Asia and South America [51] , other continents such as Oceania are also cultivating the food crop [52,53]. Global production is estimated to be highest in Africa and least in Oceania (Figure 3), but it is on the increase in the latter [53]. Thus, there is evidence of cassava being globally available for food and industrial utilization. What needs to be emphasized is the implication that if appreciated and integrated into diabetes management, either in medical nutrition therapy or pharmaceutical preparation of the dietary fibres, access to the raw material is worldwide.

Figure 3. Cassava production in the world as at 20years ago [53].
Cassava is the third most important source of calories in the tropical Africa, after rice and maize. Millions of people depend on cassava in Africa, Asia and Latin America for their source of livelihood. The bulk of world trade in cassava is in the form of pellets and chips for feed (70 percent) and the balance mostly in starch and flour for food processing and industrial use. Only a relatively small part of world production of cassava is traded internationally. It is estimated that cassava ranks as the 850th most traded, and the 1192nd most complex product. While export value is worth almost a billion dollars (US$972 million) [54], it may be interesting to note two points from Figure 4 that:
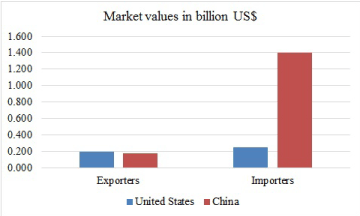
Figure 4. Market values of cassava in China and United States [54]
- Africa, especially Nigeria is the largest producer of cassava, but yet to be among the top five exporters
- China and the United State swap first and second ranks topmost importers and exporters of cassava (Figure 4). That is, these populous countries are producing cassava and consuming.
Extractability of dietary fibre from cassava: implication for supplement production
Based on the foregoing discussion, the facts about dietary fibre in cassava is arguably no more a gainsaying. Perhaps, what needs to be emphasized is how to develop the MNT potentials of the food crop. Four salient points need to be highlighted in this regard
- Studies on antidiabetic plants have yet to include cassava [1,36,55,56]. Therefore, the concept of producing dietary fibre supplement from cassava may be of interest, in addition to advancing the health value of the carbohydrate food
- Supplement or extracts of naturally-occurring dietary fibre lowers plasma LDL, VLDL-cholesterol and triglycerides and blood glucose [7,57]. The implication of this is that supplements of dietary fibre can be, and are being made from food crops.
- Cassava has become an alternative food source in poultry industry, but ironically, one of its limitation in the field is the high fibre content [58]. That is, the fibre content of cassava is being wasted in some industries
- The dietary fibres in cassava include uronic acid, pectin and β-glucans [22]. There are several reference on extraction of these nutrients from food products [59-65]. While this short paper is not focused on the details procedure of extraction or supplement preparation, it highlights the potential to enhance the agricultural and medical nutritional values of cassava.
This brief commentary has employed several references to buttress the facts about dietary fibre in cassava, is one of the macromolecule that have medicinal values. There is no gainsaying that dietary fibres content of cassava has yet to be appreciated in several studies on antidiabetic food crops. What this commentary advocates is that soluble fibres in cassava have nutraceutical values including hypocholesterolemic and hypoglycemic effects needed in diabetes management. Given the knowledge that the fibres can be extracted to produce supplement of naturally-occurring dietary fibre lowers plasma LDL, VLDL-cholesterol and triglycerides and blood glucose, it behooves to improve the agricultural economics and medical nutritional values of cassava.
All authors have contributed to this work. EUN conceptualized the work and drafted the manuscript with BCO. RJC reviewed the agricultural economics concept and the manuscript.
This work is part of doctorate research literature, which in turn is a piece of work in the 2nd phase of CVD risk assessment in prediabetes and undiagnosed diabetes mellitus study. The commentary is an extension of presentation done at the 6th International Conference on Research in Chemical, Agricultural & Biological Sciences (RCABS-2017) in Singapore 2017.
- Ebuehi OAT, Babalola O, Ahmed Z (2005) Phytochemical, nutritive and anti-nutritive composition of cassava (Manihot esculenta L) tubers and leaves. Nigerian Food Journal 23: 40-46.
- Fasanmade AA, Anyakudo MMC (2007) Glycemic indices of selected Nigerian flour meal products in male type 2 diabetes subjects. Diabetologia Croatica 36: 33-38.
- Ahmed SM, Clasen ME, Donnelly JE (1998) Management of dyslipidemia in adults. Am Fam Physician 57: 2192-2204, 2207-8. [Crossref]
- Eyambem E (2014) Wheat flour, meal/ fufu from scratch Wives Connection [cited 14th Feb 2017]; Available from: http://www.wivestownhallconnection.com/2014/10/wheat-flour-meal-fufu-from-scratch.html
- Olamijulo S (2012) Furore over cassava flour, diabetes link. Yoruba Affairs (online) [cited 29th Sep 2016]; Available from: https://groups.google.com/forum/#!topic/yorubaaffairs/CovRZB7KlV8
- Food Standards Australia New Zealand. AUSNUT 2011-13 (2016) Food nutrient database. AUSNUT 2011–13 [cited 27th Feb 2017]; Available from: http://www.foodstandards.gov.au/science/monitoringnutrients/ausnut/pages/default.aspx
- Lambeau KV, McRorie JW Jr (2017) Fiber supplements and clinically proven health benefits: How to recognize and recommend an effective fiber therapy. Journal of the American Association of Nurse Practitioners 29: 216-223.
- Nwose EU, Onodu BC, Anyasodor AE, Sedowo MO, Okuzor JN, et al. (2017) Ethnopharmacological values of cassava and its potential for diabetes and dyslipidemia management: Knowledge survey and critical review of report. J Intercult Ethnopharmacol 6: 260-266.
- Mozaffarian RS, Lee RM, Kennedy MA, Ludwig DS, Mozaffarian D, et al. (2013) Identifying whole grain foods: a comparison of different approaches for selecting more healthful whole grain products. Public Health Nutr 16: 2255-64. [Crossref]
- The National Academy of Sciences (2002) Summary. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. The National Academies Press, pp: 1-20.
- Onodu BC, Culas RJ, Nwose EU (2017) Health values of cassava compared to wheat and yam: A critical review of carbohydrate/fibre and fat/fibre ratios. In: Logotheti A, Babu GA, Seo D (eds.) 6th International Conference on Research in Chemical, Agricultural & Biological Sciences (RCABS-2017) Singapore, pp: 180-2.
- Oluwamukomi MO (2015) Chemical and sensory properties of gari enriched with sesame seed flour (Sesamum indicum L). FUTA Journal of Research in Sciences 1: 123-131.
- Patel DK, Prasad SK, Kumar R, Hemalatha S (2012) An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pacific Journal of Tropical Biomedicine 2: 320-30.
- Trinidad TP, Sagum RS, Mallillin2021 Copyright OAT. All rights reserv013) Sweet Potato and Cassava Can Modify Cholesterol Profile in Humans with Moderately Raised Serum Cholesterol Levels. Food and Nutrition Sciences 4: 491-495.
- Udenta AE, Obizoba IC, Oguntibeju OO (2014) Anti-Diabetic Effects of Nigerian Indigenous Plant Foods/Diets. Antioxidant-antidiabetic agents and human health, pp: 9-93.
- Balandrin MF, Klocke JA, Wurtele ES, Bollinger WH (1985) Natural plant chemicals: sources of industrial and medicinal materials. Science(Washington) 228: 1154-9.
- Singh R (2015) Medicinal Plants: A Review. Journal of Plant Sciences Special Issue: Medicinal Plants; 3: 50-5.
- Okoli RI, Aigbe O, Ohaju-Obodo JO, Mensah JK (2007) Medicinal Herbs Used for Managing Some Common Ailments among Esan People of Edo State, Nigeria. Pakistan Journal of Nutrition 6: 490-496.
- Vinik AI, Jenkins DJ (1988) Dietary fiber in management of diabetes. Diabetes Care 11: 160-173. [Crossref]
- Silva FM, Kramer CK, de Almeida JC, Steemburgo T, Gross JL, et al. (2013) Fiber intake and glycemic control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials. Nutrition reviews 71: 790-801. [Crossref]
- Nader N, Weaver A, Eckert S, Lteif A (2014) Effects of fiber supplementation on glycemic excursions and incidence of hypoglycemia in children with type 1 diabetes. International Journal of Pediatric Endocrinology 2014: 13.
- Post RE, Mainous AG, 3rd, King DE, Simpson KN (2012) Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. JABFM 25: 16-23.
- Tovar J, Bjorck I, Asp NG, University of Lund (1989) On the nutritional properties of starch and dietary fiber in cassava bread. [cited 6th March 2018]; Available from: http://agris.fao.org/agris-search/search.do?recordID=US19890147444
- Eleazu CO (2016) The concept of low glycemic index and glycemic load foods as panacea for type 2 diabetes mellitus; prospects, challenges and solutions. African health sciences 16: 468-79.
- Eleazu CO, Eleazu KC, Iroaganachi MA (2016) Effect of cocoyam (Colocasia esculenta), unripe plantain (Musa paradisiaca) or their combination on glycated hemoglobin, lipogenic enzymes, and lipid metabolism of streptozotocin-induced diabetic rats. Pharm Biol 54: 91-97. [Crossref]
- Bantle JP, Wylie-Rosett J, Albright AL et al. (2008) Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 31 Suppl 1: S61-S78.
- Trinidad TP, Mallillin AC, Loyola AS, Sagum RS, Encabo RR (2010) The potential health benefits of legumes as a good source of dietary fibre. Br J Nutr 103: 569-574. [Crossref]
- Jenkins DJ, Jenkins AL, Wolever TM, Vuksan V, Rao AV, et al. (1994) Low glycemic index: lente carbohydrates and physiological effects of altered food frequency. Am J Clin Nutr 59: 706s-9s. [Crossref]
- Tapsell LC (2004) Diet and metabolic syndrome: where does resistant starch fit in? J AOAC Int 87: 756-760. [Crossref]
- Bodinham C, Smith L, Thomas EL, Bell JD, Swann JR, et al. (2014) Efficacy of increased resistant starch consumption in human type 2 diabetes. Endocr Connect 3: 75-84. [Crossref]
- Ogbo FC, Okafor EN (2015) The resistant starch content of some cassava based Nigerian foods. Nigerian Food Journal 33: 29-34.
- Giavasis I (2014) Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Current opinion in biotechnology 26: 162-173.
- Pawar K, Thompkinson DK (2014) Multiple functional ingredient approach in formulating dietary supplement for management of diabetes: a review. Crit Rev Food Sci Nutr 54: 957-973.
- Callegaro MG, Diettrich T, Alves E, Milbradt BG, Denardin CC, et al. (2010) Supplementation with fiber-rich multimixtures yields a higher dietary concentration and apparent absorption of minerals in rats. Nutr Res 30: 615-625. [Crossref]
- Marinangeli CP, Jones PJ (2010) Functional food ingredients as adjunctive therapies to pharmacotherapy for treating disorders of metabolic syndrome. Annals of medicine 42: 317-33.
- Brennan CS (2005) Dietary fibre, glycaemic response, and diabetes. Mol Nutr Food Res 49: 560-570. [Crossref]
- Nwodo NJ, Nnadi CO, Ibezim A, Mbah CJ (2014) Plants with hypolipidaemic effect from Nigerian flora. Antioxidant-Antidiabetic Agents and Human Health, InTech, pp: 242-255.
- Federal Ministry of Health Nigeria (2006) Food based dietary guidelines for Nigeria: A guide to healthy living. Food and Agriculture Organization of the United Nations [cited 7th March, 2018]; Available from: http://www.fao.org/3/a-as841e.pdf
- Ene-Obong HN, Sanusi RA, Udenta EA, Williams IO, Anigo KM, et al. (2013) Data collection and assessment of commonly consumed foods and recipes in six geo-political zones in Nigeria: important for the development of a National Food Composition Database and Dietary Assessment. Food Chem 140: 539-546. [Crossref]
- Nassar NM (2006) Cassava in South America, Brazil's contribution and the lesson to be learned from India. GMR 5: 688-695.
- Welch RM (2002) Breeding strategies for biofortified staple plant foods to reduce micronutrient malnutrition globally. J Nutr 132: 495s-499s.
- Kerac M, Postels DG, Mallewa M, Alusine Jalloh A, Voskuijl WP, et al. (2014) The interaction of malnutrition and neurologic disability in Africa. Semin Pediatr Neurol 21: 42-49. [Crossref]
- Ariffin WA, Choo KE, Karnaneedi S (1992) Cassava (ubi kayu) poisoning in children. Med J Malaysia 47: 231-234. [Crossref]
- Kouame AC, Kouassi KN, N'Dri Y D, Amani NG (2015) Glycaemic index and load values tested in normoglycemic adults for five staple foodstuffs: pounded yam, pounded cassava-plantain, placali, attieke and maize meal stiff porridge. Nutrients 7: 1267-1281.
- Osagie AU, Omoregie ES (2011) The Nigeria high glycemic index starchy foods, obesity, and the environment. Nigerian quarterly journal of hospital medicine 21: 290-293.
- Eli-Cophie D, Agbenorhevi JK, Annan RA (2016) Glycemic index of some local staples in Ghana. Food Sci Nutr 5: 131-138. [Crossref]
- Ihediohanma NC (2011) Determination of the glycemic indices of three different cassava granules (Garri) and the effect of fermentation period on their glycemic responses. Pakistan Journal of Nutrition 10: 6-9.
- Trinidad TP, Sagum RS, Mallillin AC, Borlagdan MS, de Leon MP, et al. (2013) Sweet potato and cassava can modify cholesterol profile in humans with moderately raised serum cholesterol levels. Food and Nutrition Sciences 4: 491-495.
- Osipitan AA, Sangowusi VT, Lawal OI, Popoola KO (2015) Correlation of chemical compositions of cassava varieties to their resistance to Prostephanus truncatus Horn (Coleoptera: Bostrichidae). Journal of Insect Science 15: 173.
- Pinto-Zevallos DM, Pareja M, Ambrogi BG (2016) Current knowledge and future research perspectives on cassava (Manihot esculenta Crantz) chemical defenses: An agroecological view. Phytochemistry 130: 10-21.
- Chen J, Li X (2007) Hypolipidemic effect of flavonoids from mulberry leaves in triton WR-1339 induced hyperlipidemic mice. Asia Pac J Clin Nutr 16 Suppl 1: 290-294. [Crossref]
- FAO (2015) FAO Statistical Pocketbook. Food and Agriculture Organization of the United Nations 2015.
- Bourke RM (2006) Recent research on sweetpotato and cassava in Papua New Guinea: Acta Hortic, pp: 241-246.
- Onwueme IC (2002) Cassava in Asia and the Pacific. In: Hillocks RJ, Thresh JM, Bellotti AC, eds. Cassava: Biological, Production and Utiliization: CAB International, pp: 55-65.
- The Observatory of Economic Complexity (2016) Cassava HS92. [cited 21st Mar, 2018]; Available from: https://atlas.media.mit.edu/en/profile/hs92/0714/
- Salihu Shinkafi T, Bello L, Wara Hassan S, Ali S (2015) An ethnobotanical survey of antidiabetic plants used by Hausa-Fulani tribes in Sokoto, Northwest Nigeria. J Ethnopharmacol 172: 91-99.
- Gbolade AA (2009) Inventory of antidiabetic plants in selected districts of Lagos State, Nigeria. J Ethnopharmacol 121: 135-139.
- Adamson I, Okafor C, Abu-Bakare A (1990) A supplement of Dikanut (Irvingia gabonesis) improves treatment of type II diabetics. West Afr J Med 9: 108-115.
- Morgan NK, Choct M (2016) Cassava: Nutrient composition and nutritive value in poultry diets. Animal Nutrition 2: 253-261.
- Taylor KA, Buchanan-Smith JG (1992) A colorimetric method for the quantitation of uronic acids and a specific assay for galacturonic acid. Analytical Biochemistry 201: 190-196.
- Meseguer I, Aguilar V, González MaJ, Martı́nez C (1998) Extraction and Colorimetric Quantification of Uronic Acids of the Pectic Fraction in Fruit and Vegetables. Journal of Food Composition and Analysis 11: 285-291.
- Liu L, Cao J, Huang J, Cai Y, Yao J (2010) Extraction of pectins with different degrees of esterification from mulberry branch bark. Bioresour Technol 101: 3268-3273. [Crossref]
- Wang X, Chen Q, Lü X. 2014) Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocolloids 38: 129-37.
- Adetunji LR, Adekunle A, Orsat V, Raghavan V (2017) Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocolloids 62: 239-250.
- Hosseini SS, Khodaiyan F, Yarmand MS (2016) Aqueous extraction of pectin from sour orange peel and its preliminary physicochemical properties. International Journal of Biological Macromolecules 82: 920-926.




