Abstract
In the case of invasive breast carcinomas (BC) there are extremely rare late nonregional retroperitoneal lymph node recurrences, occurring after 10 years of disease diagnosis .
We present a 32-year-old woman, who was diagnosed in 2010 with left HER2 positive invasive ductal BC- pT2N1M0. Complex treatment involving radical mastectomy with axillary dissection and complex adjuvant treatment (chemotherapy, radiotherapy, targeted therapy with trastuzumab, endocrine therapy with LHRH agonist plus tamoxifen) was conducted. After 4 years, disease progression with left supraclavicular lymph node enlargement has been manifested. After surgical resection of supraclavicular lymph node, the patohistological analysis establishes lymph metastasis from HER2 positive invasive ductal carcinoma. After 6 years of multimodal treatment, including eight chemotherapy cycles docetaxel, bilateral adnexectomy, 2 targeted agents trastuzumab/pertuzumab and endocrine therapy with aromatase inhibitor, PET/ CT visualizes a nonregional lymph recurrence of left retroperitoneal lymph nodes.
Isolated involvement of distant nodal regions is extremely uncommon. Complex therapy, including a definitive radiotherapy of retroperitoneal para-aortic lymph nodes combined with targeted therapy achieved complete remission in nonregional abdominal lymph recurrence.
Keywords
HER2 positive breast carcinoma, para-aortic lymph node metastasis, Intensity modulated radiotherapy, targeted therapy, complex treatment
Introduction
Breast cancer (BC) is well known to metastasize to the entire organs by hematological spread to such as the bone, lung, liver, and the brain. It also tends to invade through the lymphatic chains mainly to the axillary nodes or occasionally to the internal mammary nodes [1]. Regional lymph nodes of the breast include the ipsilateral axillary lymph node, ipsilateral internal breast lymph node, and ipsilateral supraclavicular lymph node, which are defined by the National Comprehensive Cancer Network [2]. Distant lymph nodes, including cervical, contralateral axillary, contralateral supraclavicular, and contralateral internal mammary lymph nodes, are nonregional lymph nodes [3]. Non-regional lymph node involvement by BC has been described earlier in mediastinum, paraaortic and pelvic lymph nodes [4]. Isolated involvement of distant nodal regions is extremely uncommon. We present a rare clinical case with HER2 positive invasive ductal BC, in which we achieved complete remission of nonregional abdominal lymph recurrence after complex therapy, including Intensity modulated radiotherapy and targeted therapy.
Clinical case
We present a 32-year-old woman who was diagnosed with invasive ductal carcinoma of the left mammary gland in 2010. The stage at diagnose was pT2N1M0, grade G3 with positive estrogen, progesterone and HER2 receptors expression. A radical mastectomy with axillary dissection, complex adjuvant treatment were conducted in the same year which included chemotherapy (Ch), radiotherapy (RT), targeted therapy with trastuzumab and endocrine therapy with LHRH agonist plus tamoxifen. In 2014 extirpation of a left supraclavicular lymph node enlargement was performed with pathohistologically verification of lymph node metastasis from HER2 positive invasive ductal carcinoma. A multimodal treatment was conducted consisted of 8 Ch cycles with docetaxel, bilateral adnexectomy, 2 targeted agents including trastuzumab/pertuzumab and endocrine therapy with aromatase inhibitor. In 2017 clinical remission has been achieved. Treatment with targeted therapy trastuzumab and pertuzumab continues (Figure 1). In August 2020, the control PET/ CT after 200 MBq 18F-FDG visualizes left retrocrural and para-aortic lymph nodes at L1 level with increased metabolic activity (Figure 2).
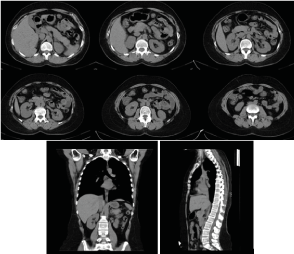
Figure 1: Axial CT of the abdomen, sagittal and coronary reconstructions of the abdomen and chest with a normal CT scan/ November 2019.
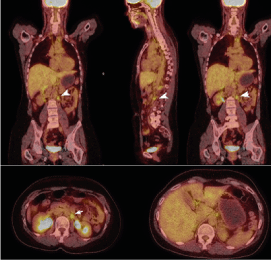
Figure 2: PET/ CT/ August 2020- Left retrocrural and para-aortic lymph nodes at L1 level with increased mitobolic activity (White arrow shows metastatic lymph nodes)
Oncology Commission considers the complex treatment to continue with a definitive radiotherapy of the para-aortic lymph nodes combined with trastuzumab target therapy. In September 2020, we conducted an intensity modulated radiotherapy (IMRT) using the VMAT method in the field of para-aortic lymph nodes and left internal and external pelvic lymph nodes with daily dose (DD) 1.8 Gy up to total dose (TD) 54 Gy (Figure 3, Figure 4). After 1 year of IMRT combined with targeted therapy, including trastuzumab and pertuzumab, a single metabolic active left retroperitoneal lymph node was displayed (Figure 5). The patient continued targeted therapy with trastuzumab and lapatinib. After the complex treatment of retroperitoneal lymph nodes at a control PET/CT from 10.01.2022, a complete therapeutic response was reported (Figure 6). Conclusion of PET/CT: There are no data on local relapse in the left breast. No new metastatic metabolic active lesions in the organ structures are established. The patient continued targeted therapy with trastuzumab and lapatinib.
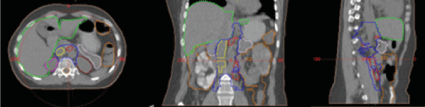
Figure 3: Contouring of target volumes and normal adjacent organs as preparation for radiotherapy planning.

Figure 4: Intensity modulated radiotherapy (IMRT) using the VMAT method in the field of para-aortic lymph nodes and left internal and external pelvic lymph nodes with DD 1.8 Gy up to TD 54 Gy. Dose distribution in target volumes and normal adjacent organs.
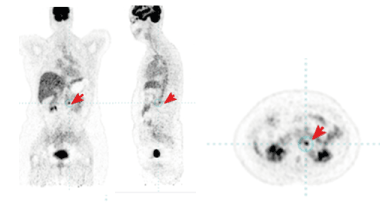
Figure 5: PET/CT from August 2021 - After 1 year of IMRT combined with target therapy, including trastuzumab and pertuzumab, a single metabolic active left retroperitoneal lymph node was displayed. The red arrow shows the metastatic lymph node.
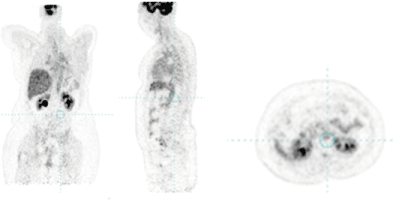
Figure 6: PET/CT from 10.01.2022 with a complete therapeutic response.
Discussion
The external axillary drainage can also be found in 20-27% of BC cases, which includes the ipsilateral internal mammary chain (17%), intramammary (3%), interpectoral (2%), and supraclavicular (2%) nodes [5]. There have been accumulating knowledges that the lymphatic pathways are relatively easy to be altered after axillary lymph node (LN) dissection and/or radiation into the contralateral axilla [6,7], paravertebral [8], or epigastric nodes [9].
Distant nonregional lymph node metastasis (DLNM) includes simultaneous and synchronous metastasis. The former indicates that metastasis existed at the initial diagnosis of breast cancer, and the latter indicates that DLNM took the form of recurrence after treatment. The two modalities are similar in proportion [10]. DLNM may be caused by the diversion and retrogradation of lymphatic drainage following the destruction of the ipsilateral lymphatic network [11-14]. Furthermore, when the original lymphatic vessels are interrupted due to surgery or radiotherapy, the lymphatic drainage may even follow collateral lymphatic channels to an alternative lymph node basin [15]. These drainage patterns could be different when compared to primary surgery and may thus result in unexpected aberrant lymphatic drainage [16]. Аberrant lymphatic spread may occasionally occur especially after occlusion of conventional pathways of lymphatic drainage following axillary dissection or irradiation [17,18]. Lymphatic mapping seems feasible after previous BCT with axillary treatment, in spite of a relatively low identification rate [19]. Aberrant drainage patterns tend to be visualised more frequent in the group of patients after previous axillary lymph node dissection (ALND) [20].
In the clinical case presented, 6 years prior to the performance of retroperitoneal metastasis, surgical extirpation of a large supraclavicular lymph nodes with pathochistological result metastases from invasive ductal carcinoma was carried out. This surgical intervention has led to a change in lymph vessels, which we believe is the cause of para-aortic lymph node metastasis. Borst et al. reported that peritoneal and retroperitoneal metastases occurred in 0.6% of ductal carcinoma and 3.1% of lobular carcinoma cases [21]. There were 6 patients with bilateral and 4 patients with unilateral hydronephrosis. The causes of hydronephrosis were retroperitoneal metastasis in 2 cases, lymph node metastasis in 2 cases, and bladder metastasis in 6 cases. However, Guru et al. considered that the prognosis of DLNM patients is similar to that of breast cancer patients with oligometastasis [22]. Several lines of recent clinical evidence support that LN metastasis plays a significant role in systemic dissemination of cancer cells, although the effect of surgical LN dissection on survival was downplayed historically because of controversial data [23]. William Halsted proposed that breast cancer is a local disease that spreads primarily in a predictable, stepwise manner from the primary tumor to the regional lymphatics and then systemically to distant organs [24]. According to the “spectrum” theory, сancer cells develop metastatic potential as tumors grow through their clinical evolution. Accordingly, the LN involvement is of prognostic importance not only because it indicates a more malignant tumor biology, but also because persistent disease in LNs can be the source of subsequent fatal metastases [25]. For diagnosis and reporting of the tumor response, PET/CT is required, which can track in the dynamics the condition of the engaged lymph nodes (Figure 2, Figure 5). In the clinical case, we have achieved a partial healing response after 1 year of radiotherapy combined with target therapy (Figure 5) and a complete therapeutic response after 1.5 year of complex treatment (Figure 6).
Conclusion
The metastases in nonregional lymph nodes for breast carcinoma are extremely rare. Surgical interventions in the field of axillary or supraclavicular lymph nodes alter the original lymph runoff and create conditions for the growth of abnormal lymph vessels contributing to late distant lymph recurrences. The forecast is the same as in distant oligometastases. Complex treatment involves radical operation, radical radiation doses, chemotherapy and targeted therapy.
Isolated involvement of distant nodal regions is extremely uncommon. In such rarely diagnosed clinical cases require a complex treatment involving a definitive radiotherapy of retroperitoneal para-aortic lymph nodes combined with targeted therapy. This therapeutic approach allows the achievement of complete remission in nonregional abdominal lymph node recurrence.
References
- Kimoto T, Kohno N, Okamoto A, Ota K, Tani T, et al. (2021) A case of contralateral inguinal lymph node metastases from breast cancer. Surg Case Rep 7: 99. [Crossref]
- Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, et al. (2018) Breast cancer, version 4.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 16: 310-320. [Crossref]
- Lin H, Lin J, Wu Y, Liang G, Sun J, et al. (2022) Exploring the Prognosis of Breast Cancer with Synchronous Distant Nonregional Lymph Node Metastasis and Establishing a Predictive Model: A Population-Based Study. Biomed Res Int 2022: 5027457. [Crossref]
- Goyal S, Puri T, Julka PK (2015) Breast cancer with inguinal node recurrence. J Egypt Natl Canc Inst 27: 41-43. [Crossref]
- Jansen L, Doting MH, Rutgers EJ, de Vries J, Olmos RA, et al. (2002) Clinical relevance of sentinel lymph nodes outside the axilla in patients with breast cancer. Br J Surg 87: 920-925. [Crossref]
- Kaur P, Kiluk JV, Maeda T, Ramos D, Koeppel W, et al. (2011) Sentinel lymph node biopsy in patients with previous ipsilateral complete axillary lymph node dissection. Ann Surg Oncol 18: 727-732. [Crossref]
- Sato A, Sakai T, Iwase T, et al. (2019) Altered lymphatic drainage patterns in re-operative sentinel lymph node biopsy for ipsilateral breast tumor recurrence. Radiat Oncol 14: 159-165. [Crossref]
- Richard R, Pereira Arias-Bouda LM, Valdés Olmos RA (2019) Unexpected lymphatic drainage of the treated breast. Clin Nucl Med 44: 732-734. [Crossref]
- Milardovic R, Castellon I, Mills C, Altinyay ME, Raphael B, et al. (2006) Scintigraphic visualization of an epigastric sentinel node in recurrent breast cancer after lumpectomy and postoperative radiation therapy. Clin Nucl Med 31: 207-208. [Crossref]
- Morcos B, Jaradat I, El-Ghanem M (2011) Characteristics of and therapeutic options for contralateral axillary lymph node metastasis in breast cancer. Eur J Surg Oncol 37: 418-421. [Crossref]
- Barranger E, Montravers F, Kerrou K, Marpeau O, Raileanu I, et al. (2004) Contralateral axillary sentinel lymph node drainage in breast cancer: a case report. J Surg Oncol 86: 167-169. [Crossref]
- Chkheidze R, Sanders MAG, Haley B, Leitch AM, Sahoo S (2018) Isolated contralateral axillary lymph node involvement in breast cancer represents a locally advanced disease not distant metastases. Clin Breast Cancer 18: 298-304. [Crossref]
- Uçmak Vural G, Şahiner I, Demirtaş S, Efetürk H, Demirel BB (2015) Sentinel lymph node detection in contralateral axilla at initial presentation of a breast cancer patient: case report. Mol Imaging Radionucl Ther 24: 90-93. [Crossref]
- Vicente JS, Domínguez Grande ML, Barquero CD, et al. (2011) Bilateral axillary and internal mammary drainage in breast cancer without prior surgery during sentinel node mapping. Indian J Nuclear Med 26: 205-207. [Crossref]
- Morcos B, Jaradat I, El-Ghanem M (2011) Characteristics of and therapeutic options for contralateral axillary lymph node metastasis in breast cancer. Eur J Surg Oncol 37: 418-421. [Crossref]
- Rees WV, Robinson DS, Holmes EC, Morton DL (1980) Altered lymphatic drainage following lymphadenectomy. Cancer 45: 3045-3049.
- Sood A, Youssef IM, Heiba SI, El-Zeftawy H, Axelrod D, et al. (2004) Alternative lymphatic pathway after previous axillary node dissection in recurrent/primary breast cancer. Clin Nucl Med 29: 698-702. [Crossref]
- Wellner R, Dave J, Kim U, Menes TS (2007) Altered lymphatic drainage after breast-conserving surgery and axillary node dissection: local recurrence with contralateral intramammary nodal metastases. Clin Breast Cancer 7: 486-488. [Crossref]
- Goyal S, Puri T, Julka PK (2015) Breast cancer with inguinal node recurrence. J Egypt Natl Canc Inst 27: 41-43. [Crossref]
- Maaskant-Braat AJ, de Bruijn SZ, Woensdregt K, Pijpers H, Voogd AC, et al. (2012) Lymphatic mapping after previous breast surgery. Breast 21: 444-448. [Crossref]
- Borst MJ, Ingold JA (1993) Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery 114: 637-641. [Crossref]
- Guru SD, Loprinzi CL, Yan E, Hoskin TL, Jakub JW (2019) Contralateral axillary metastases in breast cancer: stage IV disease or a locoregional event? Am Surg 85: 1391-1396. [Crossref]
- Kawada K, Taketo MM (2011) Significance and Mechanism of Lymph Node Metastasis in Cancer Progression. Cancer Res 71: 1214-1218. [Crossref]
- Halsted WS (1907) The results of radical operations for the cure of carcinoma of the breast. Ann Surg 46: 1-19. [Crossref]
- Hellman S (1994) Karnofsky memorial lecture: natural history of small breast cancers. J Clin Oncol 12: 2229-2234. [Crossref]





