Essiac® is an herbal compound that has been widely used as a dietary supplement for health and immune system support, as well as a homeopathic cancer treatment. Despite multiple studies aiming to demonstrate its touted benefits, the results have been inconclusive. Some studies have shown Essiac® to impart gastroenterological protection, combat reactive oxygen species (ROS), increase immune cell subsets, and reduce in vitro cancer cell numbers, while other studies have not been able to show reduced cancer load in vivo. Therefore, in this study using the fully-prepared proprietary blend, Essiac® Liquid Herbal Extract (LHE), we thoroughly explored its health benefits using the nematode animal model, Caenorhabditis elegans (C. elegans), as well as assessed its antiproliferative abilities against three non-adherent (myeloma, lymphoma, and leukemia) and two adherent tumor forming (breast and prostate) cancer cell lines. Our findings show that when C. elegans were exposed to the recommended dosage of Essiac® LHE, there was an increase in their overall lifespan, and an increase in their ability to withstand oxidative stress induced mortality when challenged. Additionally, our work demonstrated that a 24% exposure of Essiac® LHE induced a significant decrease in cell viability and proliferation within all five cancer cell lines (RPMI 8226, Jurkat, CML, LNCaP, and MCF7). Furthermore, our results indicate that the anti-proliferative effects of Essiac® LHE are not being mediated through the induction of intrinsic apoptosis, but through an alternative cellular mechanism. Taken together, these in vitro and in vivo findings lend support to the overall health benefits and antiproliferative abilities of Essiac® LHE.
Essiac®, oxidative stress, cancer, C. elegans, herbal medicine, complementary and alternative medicine
C. elegans: Caenorhabditis elegans; CAM: Complementary and alternative medicine; CML: Chronic Myeloid Leukemia; ER: Estrogen receptor; LHE: Liquid herbal extract; PS: Phosphatidylserine; ROS: Reactive oxygen species
The use of Complementary and Alternative Medicine (CAM), which include practices such as traditional-indigenous medicinal practices and herbal supplements [1], continues to grow rapidly around the world [2,3], and is particularly popular amongst cancer patients, with approximately 87% reporting some CAM use [4-7]. For more than 90 years, Essiac®, a popular North American CAM [8,9], has been widely used based on its testimonial claims of improving the immune system, reducing cancer load, and improving overall quality of life [10] with very few side effects (mild nausea and diarrhea) [11]. Essiac®, is a proprietary blend of four herbs: sheep sorrel (Rumex acetosella), burdock root (Arctium lappa), slippery elm bark (Ulcus fulva), and Indian rhubarb root (Rheum palmatum), derived from an Ojibwe Indian remedy used for both spiritual balance and body healing [12]. Individually, each of the four herbs have been shown to exhibit health benefits through their expansive chemical components such as vitamins, minerals, organic acids, phytoestrogens, anthraquinones, and many antioxidants [13]. Particularly, these herbs have been shown to have antioxidant, anti-inflammatory, immunomodulatory, antitumor, anticancer, antibacterial, diuretic, laxative, wound healing, and gastroprotective properties [11,14,15]. In vitro studies exploring Essiac’s® health benefits have also demonstrated it to have strong antioxidant abilities [14-16], anti-inflammatory [16] and immunomodulatory activity [15], and antiproliferative properties against cancer cells [15,17,18], although one study revealed increased breast cancer cell proliferation at low exposures [19].
To date, all efforts investigating the in vivo health benefits of Essiac® have been inconsistent, as many of the previous studies are incomplete or non-peer reviewed, and those that are have been peer-reviewed have used various formulations or preparations of Essiac®, all which has led to inconclusive evidence [11,20,21]. There is also a dearth of studies using the direct fully-prepared proprietary blend, Essiac® Liquid Herbal Extract (Essiac® LHE). Despite this, Essiac® continues to remain a widely popular CAM treatment. Therefore, this study aimed to contribute to the existing body of evidence by conducting a more thorough assessment of the health, antioxidant, and anti-proliferative abilities of Essiac® LHE, using both in vivo and in vitro models. We hypothesized that the high antioxidant capacity of Essiac® LHE would improve the overall health and the ability to withstand oxidative stress in Caenorhabditis elegans (C. elegans), a nematode animal model that has been widely used to assess lifespan, oxidative stress, and innate immunity. The relatively short lifespan (2-3 weeks) and inducible innate immune system make C. elegans an appropriate model for this study [22-25]. Additionally, because previous studies investigating the anti-cancer effects of Essiac® have been inconclusive, in this study we sought to more comprehensively investigate the antiproliferative ability of Essiac® LHE exposure on: two adherent/tumor forming cell lines previously used in Essiac® studies (breast and prostate), and three non-adherent/non-tumor forming cancer cell lines (T cell leukemia; B-cell myeloma and Chronic Myeloid Leukemia). The latter two non-adherent/non-tumor forming cancer cell lines listed have not been previously explored in the context of Essiac®. We hypothesized that the high antioxidant and anthraquinone content found within the herbs of Essiac® LHE would induce both anti-proliferative and cytotoxic effects in the various cancer cell lines (MCF7, LNCaP, CML, Jurkat, and RPMI 8226).
Essiac® liquid herbal extract (LHE)
Essiac® Liquid Herbal Extract (Essiac® Canada International Inc., Ottawa, ON, Canada) was purchased from Amazon.com. Upon arrival, Essiac® LHE bottles were kept at room temperature, and stored at 4°C upon opening for up to two weeks, as indicated by the manufacturer. The suggested human dosage is 40 mL daily.
Maintenance of C. elegans and Essiac® LHE exposure
Wildtype (N2 Bristol) C. elegans were obtained from the Caenorhabditis Genetic Center (University of Minnesota, Minneapolis, MN, USA) and maintained as frozen stock until needed. All synchronized cultures were grown on 60 mm solid NGM plates seeded with a spot (100 mL) of OP50 E. coli for nutrients at 22°C. Reproductive adults were placed onto fresh 60 mm NGM plates and allowed to lay eggs for 2-4 hours, producing age-synchronized groups. Working cultures were maintained at 22°C. For all experimental exposures to Essiac® LHE, 35 mm plates were seeded with 50 mL of OP50 that contained various concentrations of the suggested dosage of Essiac® LHE. For experimental exposures, the manufacturer recommended daily human dosage of Essiac® LHE (40 mL) was added to 10 mL of fresh OP50 (which had been grown overnight shaking at 37°C), and this constituted a 100% Essiac® LHE exposure treatment. A serial dilution was then used to get the remaining dosage concentrations (50%, 25%, 12.5%, 6.25%, 3.125%, and 1.5625%). Control groups were exposed to OP50 alone.
Health/longevity assay
The health and longevity of C. elegans was assessed with a longevity assay. Synchronized worms at three days of age (L4 stage) were transferred to 35 mm plates seeded with either 50 mL of Essiac® LHE in OP50 at the specified dosage or control (OP50 alone), and maintained at 22°C. Each group contained a total of 100 worms over four plates, per assay. To avoid confounding generations, the original worms were transferred every two days to fresh plates until they stopped producing eggs. Worms were checked and recorded daily for survival for the next 28 days, with removal of dead worms. Worms were scored as dead if they did not respond to a touch stimulus. A total of five trials were conducted for each dosage exposure (3.125%, 6.25%, 12.5% and 25%, 50%, and 100%).
Acute oxidative stress assay
The ability of C. elegans to withstand oxidative stress was assessed through an acute oxidative stress assay. Synchronized worms at three days of age (L4 stage) were transferred to 35 mm plates seeded with 50 mL of Essiac® LHE in OP50 at 100% dosage or control (OP50 alone), for 48 hours, after which they were transferred to 35 mm plates containing 472 mM juglone (5-hydroxyl-1, 4-naphthoquinone, Sigma Aldrich) within the NGM [26]. Juglone is a quinone that generates superoxide anion (O2-) from molecular oxygen during metabolism. The plates were prepared by dissolving juglone in 100% ethanol and immediately mixing it into liquefied NGM at 55°C for a final concentration of 472 mM. The mixture was then poured into 35 mm plates. After solidification, the plates were seeded with 20 mL of OP50 and allowed to dry in a fume hood for 30 min, after which a ring of palmitic acid (10 mg/mL in ethanol) was applied to the edge of the NGM to inhibit worms from crawling along the sides of the plate. Juglone plates were used as soon as they were prepared, transferring the treated and control groups to their respective plates, and monitored for mortality every 30 min for a four-hour period. Worms were scored as dead if they did not respond to a touch stimulus. Each group contained a total of 100 worms over four plates, per assay. A total of three trials were conducted for this assay.
Maintenance of cell lines and Essiac® LHE exposure
All cell lines were obtained from ATCC and maintained at 37°C with 5% CO2. Breast cancer adherent cell line MCF7 (HTB-22) was cultured in Eagle’s MEM medium with 10% FBS and 0.01 mg/mL of human recombinant insulin. Chronic myeloid leukemia non-adherent cell line CML (K-562, CCL-243) was cultured in Iscove’s MDM medium with 10% FBS. Prostate cancer adherent cell line LNCaP (clone FGC, CRL-1740), B-cell myeloma non-adherent cell line RPMI-8226 (CCL-155), and T-cell leukemia non-adherent cell line Jurkat (clone E6-1, TIB-152) were all cultured in RPMI-1640 media with 10% FBS. For all experimental exposures, Essiac® LHE was directly mixed into the respective medium at the desired final concentration (1.5%, 3%, 6%, 6.25%, 12%, 12.5%, 24%, 25%, 48%, and 50%). Control groups were exposed to medium alone.
Trypan blue exclusion assay
Cell viability was assessed through trypan blue (Millipore-Sigma-Aldrich, USA) exclusion. This dye enters disrupted cell membranes (e.g., dead cells) marking them blue. In contrast, living cells with intact membranes exclude dye entry marking them clear/white. For this assay, RPMI 8226 cells were exposed to various concentrations of Essiac® LHE (6.25%, 12.5%, 25%, and 50%) mixed directly into the cell medium. Control groups were exposed to medium alone. Using a 6-well plate, each group consisted of 200,000 cells/well, in triplicate, in a total volume of 2 mL/well. Once the cells were placed into a 6-well plate, they were incubated overnight at 37°C with 5% CO2 to acclimate. The next day experimental groups received Essiac® LHE at the specified concentration, or additional medium alone for controls, and incubated for 48 hours at 37°C with 5% CO2. After the exposure period, cells were suspended within their wells and a 10 mL sample was collected and mixed with 10 mL of 0.4% trypan blue, after which 10 mL of this mixture was applied to a slide chamber and read for cell viability and death in a LUNA-FL cell counter (Logos Biosystems). A total of five trials were conducted for this assay.
MTT cell proliferation assay
Cell viability and proliferation was assessed with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) MTT assay [27]. For this assay we used adherent cell lines (LNCaP and MCF7), and non-adherent cell lines (Jurkat and CML). We added 20,000 cells/well into a 96-well plate, in triplicate, gently rocked the plate back and forth, and incubated it overnight at 37°C with 5% CO2. The following day, groups were exposed to Essiac® LHE at test concentrations (1.5%, 3%, 6%, 12%, 24%, and 48%) or medium alone (control), rocked gently to mix, and left to incubate for an additional 48 hours at 37°C with 5% CO2. At the end of 48 hours, the plate was centrifuged at 1000 rpm for five minutes. Next, old medium was discarded from each well and 100 mL of fresh medium was added along with 10 mL of 12 mM MTT (#M6494, ThermoFisher, USA) solution. The plate was then incubated for an additional four hours at 37°C with 5% CO2 after which it was centrifuged again (1000 rpms, 5 min) and 85 mL of medium was removed from each well. Next, 50 mL of dimethyl sulfoxide (DMSO) was added and mixed thoroughly in each well and incubated for an additional 10 min at 37°C with 5% CO2. Samples in each well were mixed again after the incubation period and absorbances were read at 540 nm with a BioTek Synergy HT plate reader (Agilent, Vermont, USA). A total of three trials were conducted for this assay.
CellTiter 96® AQueous assay
Cell viability and proliferation was assessed with the CellTiter 96® AQueous Non-Radioactive Cell Proliferation assay (#G5421, Promega, USA), a more sensitive assay, as instructed by the manufacturer [28]. For this assay we used adherent cell lines (LNCaP and MCF7), and non-adherent cell lines (Jurkat and CML). We added 10,000 cells/well into a 96-well plate, in triplicate, and then gently rocked the plate back and forth and incubated it overnight at 37°C with 5% CO2. The following day, groups were exposed to Essiac® LHE at test concentrations (1.5%, 3%, 6%, 12%, 24%, and 48%) or medium alone (control), rocked gently to mix, and left to incubate for an additional 48 or 72 hours at 37°C with 5% CO2. At the end of the 48 or 72 hour incubation, 20 mL of MTS/PMS solution was added to each well, mixed gently, and incubated for an additional 3.5 hours at 37°C with 5% CO2. At the end of the incubation period, the absorbances were read at 490 nm with a BioTek Synergy HT plate reader (Agilent, Vermont, USA). A total of four trials were conducted for this assay.
Caspase-Glo® 3/7 assay
Caspase activity was assessed with the Caspase-Glo® 3/7 assay (#G8090, Promega, USA), as instructed by the manufacturer. For this assay we used adherent cell lines (LNCaP and MCF7), and non-adherent cell lines (Jurkat and CML). We added 20,000 cells/well into a white 96-well plate, in triplicate, and then gently rocked the plate back and forth and incubated it overnight at 37°C with 5% CO2. The following day, groups were exposed to 24% Essiac® LHE or medium alone (control), rocked gently to mix, and then incubated at 37°C with 5% CO2 for another 48 hours. After the 48-hour incubation, each well received 100 mL of Caspase-Glo® 3/7 reagent, was mixed gently, incubated in the dark at room temperature for one hour, and then read for luminescence on a Synergy HT plate reader (Agilent, Vermont, USA). Luminescence is proportional to the amount of caspase activity. A total of three trials were conducted for this assay.
RealTime-GloTM annexin V apoptosis and necrosis assay
Apoptosis and secondary necrosis were assessed with the RealTime-GloTM Annexin V Apoptosis and Necrosis assay (#JA1101, Promega, USA), as instructed by the manufacturer. This assay measures the exposure of phosphatidylserine (PS) on the outer bilayer of the cell membrane during the apoptosis process [29]. Annexin V binding to PS is detected by luminescence. A cell-impermeant fluorescent DNA dye detects necrosis when there is a loss of cell membrane integrity. For this assay we used adherent cell lines (LNCaP and MCF7), and non-adherent cell lines (Jurkat and CML). We added 20,000 cells/well into a white 96-well plate, in duplicate, and then gently rocked the plate back and forth and incubated it overnight at 37°C with 5% CO2. The following day, groups were exposed to 24% Essiac® LHE or medium alone (control), rocked gently to mix, and then each well received 2X Detection Solution and incubated at 37°C with 5% CO2 for another 48 hours. During the second 48-hour incubation period, readings were taken as follows: every four hours for the first 24 hours, then every six hours for the remaining 24 hours. During each reading, fluorescence was read at 485 nm ex/525 nm em followed by a luminescence read on a Synergy HT plate reader (Agilent, Vermont, USA). A total of three trials were conducted for this assay.
Statistical analysis
All calculations and statistical analyses were conducted using GraphPad Prism9 software. A Student’s T-test with Welch’s correction was used to determine statistical differences between control and experimental groups for all assessments, except survival curves, which used the Log-Rank (Mantel-Cox) test. Results showing p≤0.05 were considered to be statistically significant.
Daily exposure to Essiac® LHE increases longevity in C. elegans
Due to the high antioxidant components found within Essiac® (Table 1), we first wanted to determine if daily exposure to Essiac® LHE had the ability to improve health in C. elegans, as assessed by their overall lifespan. In optimum conditions, C. elegans live for an average of 12-18 days [22], therefore anything impacting their health will show an effect in their overall lifespan. As the suggested daily dosage for Essiac® LHE is intended for human consumption, we exposed C. elegans to various dosages of Essiac® LHE, starting with a full dosage exposure (100%), down to a 3.125% dose using a serial dilution (Figure 1). We found that C. elegans exposed to the full daily dosage of Essiac® LHE resulted in a statistically significant increase in their overall lifespan (p=0.004), with an improved median survival of 16 days compared to 14 days in the control group (Figure 1F). The mean lifespan of worms exposed to Essiac® LHE extended out to 23 days, five days beyond the average 18-day lifespan. All groups started out with 100 worms, and at the 18-day timepoint, those treated with 100% Essiac® LHE had an average of 26 surviving worms, compared to an average of 14 survivors in the control group. All other dosage groups had a median survival of 14-15 days, comparable to their respective control groups. The results of this assessment suggest that daily consumption of a 100% dosage of Essiac® LHE by C. elegans is associated with an overall increase in their health and lifespan.
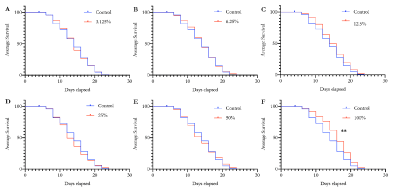
Figure 1. Essiac® LHE exposure corresponds to an increase in longevity of C. elegans. Starting at the L4 stage, C. elegans were exposed to Essiac® LHE at various concentrations, or OP50 alone (control) and assessed every two days for mortality until they all died. Worms were exposed at the following Essiac® LHE dosages: (A) 3.125%, (B) 6.25%, (C) 12.5%, (D) 25%, (E) 50%, and (F) 100%. Survival curves are representative of the average of five trials. **p<0.01
Table 1. Components found with the four herbs of Essiac® LHE
Oxidative stress induced mortality is reduced with Essiac® LHE exposure
As daily Essiac® LHE exposure was associated with increased longevity, we next wanted to determine if a short exposure could affect the ability of C. elegans to withstand oxidative stress. We exposed C. elegans to a 100% dosage of Essiac® LHE for 48 hours starting at the L4 stage of development (one day prior to reaching adult development), and then challenged them with an acute concentration of juglone (a strong inducer of reactive oxygen species (ROS) which often leads to death [26]), and assessed mortality every 30 min (Figure 2). We found that after 90 min, those exposed to Essiac® LHE showed a decrease (p=0.05) in mortality compared to the control group. The ability to withstand death induced by ROS was maintained throughout the four-hour challenge (p<0.05), showing that at each assessment timepoint, on average, the Essiac® LHE exposed group experienced 4 deaths compared to 7 in the control group. Each group started with 100 worms and at the final 240 min timepoint, the Essiac® LHE group had an average of 68 survivors compared to 40 survivors in the control group. These findings indicate that the consumption of Essiac® LHE by C. elegans increases their ability to respond against ROS induced death, likely due to the high antioxidant components of Essiac®.
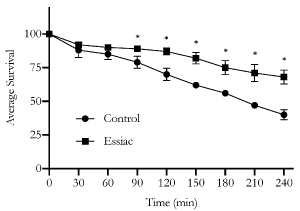
Figure 2. The ability to resist acute oxidative stress is increased with Essiac® LHE exposure. Starting at the L4 stage, C. elegans were exposed to a 100% dosage of Essiac® LHE, or OP50 alone (control) for 48 hours, and then exposed to a high dose of juglone to induce oxidative stress and monitored for mortality every 30 min for a four-hour period. The graph is representative of the average of three trials. Error bars represent that standard deviation of the average. *p≤0.05
Essiac® LHE decreases both adherent and non-adherent cancer cell proliferation
Previous in vitro studies investigating the antiproliferative properties of Essiac® against cancer cell lines have been inconclusive, have used different variations of the Essiac® preparation, and have primarily focused on adherent (tumor forming) cancers. Therefore, we wanted to assess the antiproliferative abilities of the fully-prepared Essiac® LHE using two adherent cancer cell lines (MCF-7, breast cancer and LNCaP, prostate cancer) that were previously used in other studies, and three additional non-adherent leukemia cancer cell lines, two of which have never been tested (Jurkat, RPMI 8226, and CML, respectively).
We first conducted a proof-of-principle assessment using the B-cell myeloma cancer cell line, RPMI 8226. These cells were exposed to various concentrations of Essiac® LHE for a 48-hour period, and then assessed for viability by Trypan Blue exclusion (Figure 3). This initial assessment demonstrated that exposure as low as 6.25% Essiac® LHE induced a reduction in the viability of RPMI 8226 cells (p<0.001), showing an average of 24.3% viability compared to 72.5% viability in control cells. Exposures at higher concentrations demonstrated the same reduced viability, with the 12.5% Essiac® LHE exposure showing an average of 25.7% viability, 25% Essiac® LHE exposure an average of 14.6% viability, and the 50% Essiac® LHE exposure leading to 100% cell death.
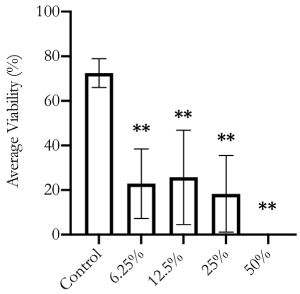
Figure 3. RPMI 8226 cell viability is reduced with Essiac® LHE exposure. Cells were exposed to various concentrations of Essiac® LHE, or medium alone (control) for 48 hours and then assessed for viability using Trypan Blue. The graph is representative of the average of three trials. Error bars represent that standard deviation of the average. **p<0.01
As Essiac® LHE was able to reduce viability in B-cell myeloma cells, we next focused on two prevalent leukemias, Chronic Myeloid Leukemia (CML, K-562) and Acute T-cell leukemia (Jurkat), and the two adherent cancer cell lines that have been previously used in Essiac® studies, breast cancer (MCF-7) and prostate cancer (LNCaP). We exposed these cell lines to various concentrations of Essiac® LHE, with the maximum concentration (48% for 48 hours) and then assessed them for viability using the MTT proliferation assay (Figure 4). We found that exposure to Essiac® LHE led to a reduction in cell proliferation of all cell lines, with the exception of Jurkat, compared to their control groups. Specifically, CML cells showed a reduction at the two highest concentrations of 24% (p=0.02) and 48% (p=0.03) (Figure 4A). LNCaP cells were very sensitive to Essiac® LHE exhibiting reduced cell proliferation at all concentrations (p<0.05) (Figure 4C). MCF-7 cells also appeared to be sensitive at the higher concentrations of 12% (p=0.05) and 24% (p=0.03), but did not seem to be affected by the highest exposure of 48% (p=0.2) (Figure 4D). It was also apparent that all groups within each cell line assessed showed high variance (error bars), but as the concentration of Essiac® LHE increased (with the exception of MCF7), the variance within each group decreased, signifying that the cells were being consistently affected at the higher concentrations.
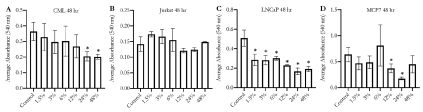
Figure 4. Essiac® LHE exposure reduces cell proliferation in both adherent and non-adherent cancer cells. (A) CML, (B) Jurkat, (C) LNCaP, and (D) MCF7 cancer cells were exposed to various concentrations of Essiac® LHE, or medium alone (control) for 48 hours and then assessed for cell proliferation by MTT assay. Graphs represent the average of three trials. Error bars represent that standard deviation of the average. **p < 0.05
To confirm the reduction in cell proliferation induced by Essiac® LHE exposure, we repeated the experiment using a more sensitive cell proliferation assay, CellTiter 96®, and included an extended exposure period of 72 hours to determine if the antiproliferative effect was maintained over time. The results of these assessments revealed that Essiac® LHE was able to stimulate a reduction in cell proliferation in all four cell lines following a 48-hour exposure period, and this reduction was maintained at the longer 72-hour exposure period (Figure 5). Interestingly, while our previous MTT cell proliferation assay did not show any impact of Essiac® LHE upon Jurkat cells after a 48-hour exposure (Figure 4B), the CellTiter 96® proliferation assay was able to reveal an effect for both exposure periods (p 0.05) (Figures 5B and 5F). As previously seen with the MTT assay, the CellTiter 96® proliferation assay demonstrated a clear reduction in variability within groups (error bars) for all cell lines as the concentration of Essiac® LHE increased. This reduction in response variability was associated with all statistical reductions in cell proliferation (p<0.05). Taken together, these findings infer that an exposure to 24% Essiac® LHE after a 48-hour period is able to reduce cell proliferation of both non-adherent and adherent cancer cell lines.
0.05) (Figures 5B and 5F). As previously seen with the MTT assay, the CellTiter 96® proliferation assay demonstrated a clear reduction in variability within groups (error bars) for all cell lines as the concentration of Essiac® LHE increased. This reduction in response variability was associated with all statistical reductions in cell proliferation (p<0.05). Taken together, these findings infer that an exposure to 24% Essiac® LHE after a 48-hour period is able to reduce cell proliferation of both non-adherent and adherent cancer cell lines.
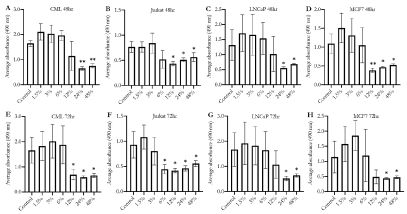
Figure 5. Higher concentrations of Essiac® LHE exposure are associated with reduced cell proliferation in both adherent and non-adherent cancer cells. (A, E) CML, (B, F) Jurkat, (C, G) LNCaP, and (D, H) MCF7 were exposed to various concentrations of Essiac® LHE, or medium alone (control) for 48 hours (A-D) or 72 hours (E-H), and then assessed for cell proliferation using the sensitive CellTiter 96® assay. The graphs are representative of the average of four trials. Error bars represent that standard deviation of the average. *p < 0.05, **p < 0.01
Essiac® LHE triggers cancer cell death by mechanisms other than apoptosis
Witnessing the antiproliferative effects of Essiac® LHE on various cancer cell lines, we next wanted to explore if the reduction in cell proliferation was the result of apoptotic cell death. Apoptosis, or intrinsic programmed cell death, is a cellular process mediated by the activation of a cascade of caspases (protease enzymes), leading to the final activation of three executioner caspases, 3, 6 and 7 [30]. Therefore, in order to determine if Essiac® LHE triggers apoptosis in cancer cells, we exposed all four cancer cell lines to 24% Essiac® LHE for 48 hours, and subsequently assessed them for caspase activity using the Caspase-Glo® 3/7 assay. Surprisingly, this assessment revealed that all four cell lines exhibited very little to barely detectable caspase activity (p<0.05) when exposed to 24% Essiac® LHE compared to their non-treated control groups (Figure 6).
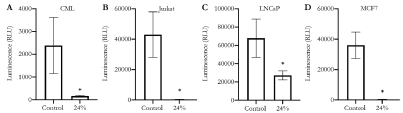
Figure 6. Essiac® LHE exposure is not associated with caspase activity in cancer cell lines. (A) CML, (B) Jurkat, (C) LNCaP, and (D) MCF7 cancer cells were exposed to 24% Essiac® LHE, or medium alone (control) for 48 hours and then assessed for caspase 3 and caspase 7 activity using the CaspaseGlo® 3/7 assay. Controls received media only. The graph is representative of the average of three trials. Error bars represent that standard deviation of the average. *p < 0.05
As we did not see any caspase activity after Essiac® LHE exposure, suggesting that the anti-proliferative effects were not being mediated through intrinsic apoptosis, we confirmed these findings by performing an additional apoptosis assay, the RealTime-GloTM Annexin V Apoptosis and Necrosis Assay. This assay measures the presence of phosphatidylserine (PS) which moves from the inner bilayer to the outer bilayer of the cell membrane during the early stages of the apoptosis process [29]. Annexin V, which is fused to luciferase, binds to PS allowing for its detection through luminescence. Additionally, a cell-impermeant fluorescent DNA dye detects necrosis when there is a loss of cell membrane integrity. Together, this system allows for the assessment of the apoptosis process over time, with the detection of early apoptosis and the induction of secondary necrosis during the later stages of apoptosis. For this assessment, we exposed all four cell lines to 24% Essiac® LHE for a 48-hour period during which we took measurements of both luminescence and fluorescence at specific time intervals. Similar to what the caspase assay demonstrated, this assay showed that for all four cell lines, apoptosis was not being induced following Essiac® LHE exposure (Figure 7). Both the luminescence and fluorescence signals followed a similar trend over the 48-hour period, indicating a non-apoptotic phenotype (Figures 7B, 7D, 7F, and 7H). In this assay, had apoptosis been occurring there would have been a clear time delay between the emergence of the luminescence signal and a subsequent rise in the fluorescence signal; this would indicate early apoptosis followed by secondary necrosis typical of late-stage apoptosis, as seen when cells are exposed to the chemotherapeutic drug cisplatin (Figures 7A, 7C, 7E, and 7G)[31]. Taken together, these two assessments indicate that the reduction in cell proliferation of cancer cells exposed to Essiac® LHE is not being mediated by apoptosis.
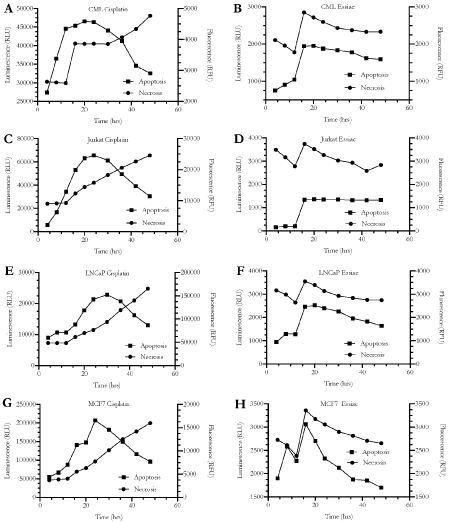
Figure 7. Reduced cancer cell viability induced by Essiac® LHE exposure is not mediated through apoptosis. (A) CML Cisplatin, (B) CML Essiac, (C) Jurkat Cisplatin, (D) Jurkat Essiac, (E) LNCaP Cisplatin, (F) LNCaP Essiac, (G) MCF7 Cisplatin, and (H) MCF7 Essiac. Cancer cells were exposed to 60 uM Cisplatin (positive control for apoptosis. A, C, E, and G) or 24% Essiac® LHE (B, D, F, and H) and assessed for the expression of phosphatidylserine (PS) over a 48-hour period using the RealTime-GloTM Annexin V Apoptosis and Necrosis assay. Annexin V fused with luciferase binds to PS indicating apoptosis (measured through luminescence). Loss of cell membrane integrity allows access to DNA, which can then be bound by a fluorescent DNA dye, used as a marker of necrosis (measured by fluorescence). The graphs are representative of the average of three trials.
Worldwide, the use of CAM continues to increase yearly. Amongst cancer patients, CAM is often used to increase a patients’ sense of control over their treatment, improve their overall health, or as a supplement with potential to augment their conventional therapy. In particular, the herbal supplement Essiac® has been a widely used CAM throughout Canada over the past 90 years, and has been gaining popularity throughout the United States and the world. Despite its popularity, to date, all efforts aimed at investigating the abilities of Essiac® have been inconsistent, leading to inconclusive evidence that supports or refutes its health and anticancer benefits. Hence, this study aimed to contribute to the body of evidence by conducting a more thorough evaluation of the health, antioxidant, and anti-proliferative abilities of the fully-prepared proprietary mixture Essiac® LHE, using both in vivo and in vitro models.
In this study we found that daily consumption of Essiac® LHE, at the manufacturer’s indicated daily dosage, led to an increase in the lifespan of the nematode animal model C. elegans (Figure 1F). The mean lifespan of worms exposed to Essiac® LHE extended out to 23 days, five days beyond the average 18-day lifespan. We also found that a short 48-hour exposure to Essiac® LHE improved the ability of C. elegans to resist oxidative stress induced death (Figure 2). It is very likely that both of these results can be attributed to the high levels of antioxidant and anthraquinones found within the Essiac® LHE herbal mixture [13]. Our findings are in line with other studies that have shown the strong antioxidant ability of various Essiac® formulations in combating different forms of ROS [14-16]. While we recognize that the results of our work conducted in C. elegans cannot be directly extended to humans, this model’s 70% genetic and 87% developmental similarities to humans [32] has justified its extensive use in diverse research endeavors spanning neurological, developmental, and toxicological fields [22,24,25,33,34]. Interestingly, the only reports of any harmful effects related to human consumption of Essiac® have been mild nausea, bad aftertaste, and diarrhea [10,21]. Therefore, we believe that our findings begin to substantiate the claims that Essiac® LHE supports overall health.
Since 1923, when Rene Cassie began treating her cancer patients with Essiac®, there have been many testimonials reporting its antitumor and anticancer properties, sparking its wide CAM use in cancer patients, most notably those with breast cancer. As such, concerted efforts investigating the potential antiproliferative effects of Essiac® have primarily focused on adherent tumor forming cancers, predominantly breast and prostate cancer [17,19,20,35]. Although one study included other cancers such as melanoma, ovarian, liver, and lung cancer [15]. Additionally, there has been just one study that included two non-adherent cancers (leukemias) [17]. Alas, the results of these few studies have been conflicting. While two studies demonstrated Essiac’s® antiproliferative effects in multiple tumor-forming cancer cell lines (LNCaP, PC3, MCF7, A549, A2780, A375, HepG2), another study showed no anticancer effects against two prostate cancer cell lines (LNCaP and PC3). These contradictory results have also been shown within one study that found that while Essiac® was able to inhibit two breast cancer cell lines (MCF7, MDA-MB-468) and acute promyelocytic leukemia (HL60), it was not able to inhibit T cell leukemia (Jurkat). Lastly, one study conducted in 2006, presented completely opposite results, finding that Essiac® increased cell proliferation in four different breast cancer cell lines (MCF7, T47D, MDA-MB-436, MDA-MB-231) [19].
Consequently, the varied findings of the aforementioned studies prompted us to conduct a more thorough exploration of the antiproliferative abilities of Essiac® LHE upon both adherent and non-adherent cancer cell lines: RMPI 8226, Jurkat, CML, MCF7 and LNCaP. Through the use of three different cell viability and proliferation assays, our study revealed that an in vitro 48-hour exposure to 24% Essiac® LHE produced a reduction in cell proliferation of all five cancer cell lines tested (Figures 3-5), with an antiproliferative effect that was maintained over a 72-hour exposure period (Figure 5). Additionally, our results indicate that the antiproliferative effects of Essiac® LHE are not mediated through the activation of apoptotic death, as demonstrated through the use of two different apoptosis assays. Together, our findings add to the body of evidence that lend support to the assertions of Essiac’s® antiproliferative and anticancer abilities [15,17,35].
Previous work that demonstrated Essiac’s® inability to inhibit cell proliferation in LNCaP, PC3 and Jurkat cancer cell lines used either a lower concentration exposure of Essiac® (10% dilution) [17], or their own preparation created from an extraction process [20], leading us to believe that these studies were simply below the concentration threshold needed to achieve full inhibition. Our own work demonstrates that a higher concentration (24%) of Essiac® LHE was necessary to produce consistent inhibition of LNCaP, Jurkat, and other cancer cell lines (Figures 3-5).
While the results of our in vitro assessments suggest that high concentrations of Essiac® LHE can be used as an antiproliferative supplement for tumor and non-tumor forming cell lines, a 2006 study conducted by Kulp et. al., cautioned against using Essiac® in hormone sensitive cancers, specifically breast cancer (MCF7), as they found that at low concentrations (1% - 8%) Essiac® increased proliferation in both estrogen receptor (ER) positive and negative breast cancer cell lines and may engage the ER [19]. In contrast, our study found that low exposures to Essiac® LHE were associated with a high variability of response within cell lines across all three cell proliferation assays, leading to inconsistent results. Furthermore, it was only at higher concentrations of (24%) Essiac® LHE that corresponded to reductions in cell proliferation (Figures 3-5).
Kulp et. al’s warning behind the use of Essiac® in breast cancer patients stems from the phytoestrogens found within the herbs of the Essiac® mixture; sheep sorrel and burdock root (13). Phytoestrogens, such as genistein and daidzein, are plant derived chemicals with structural similarity to mammalian estrogens, that have the ability to bind to ERs [36,37]. Human cells express three different types of estrogen receptors: ERa, ERb, and a membrane specific G-protein coupled ER receptor [36]. Studies have shown that signaling through ERa produces the conventional cell growth and proliferation effects typical of growth hormone engagement, while signaling through ERb has been associated with antagonistic antiproliferative effects [36,38]. Notably, multiple studies have shown that phytoestrogens primarily signal through their engagement of ERb, and that at low concentrations they can engage ERa leading to proliferation, while at higher concentrations they preferentially bind to ERβ ultimately resulting in an antiproliferative response [39-41]. Moreover, the Kulp et. al. study did not specify which ER receptors were being targeted, and the ER specific inhibitor they used (ICI 182,780) in their study to confirm that Essiac® targets ER, has been shown to bind to ERa with high affinity, with little to no binding affinity for ERb [42]. Taken together, it is clear that further research is warranted to determine the relationship Essiac® LHE may have with ER subtypes.
Collectively, the results of our study increase the evidence supporting the health and antiproliferative claims of the CAM, Essiac® LHE. Our findings demonstrate Essiac® LHE’s positive effects in relevant biological models. It has the ability to improve health and lifespan in C. elegans, while also inducing in vitro antiproliferative effects in both adherent and non-adherent cancer cell lines, potentially substantiating some of the purported health benefits of Essiac® LHE for humans. As our study also indicates that the anticancer properties of Essiac® LHE are not mediated through the induction of apoptosis, future studies should work towards elucidating the cellular mechanism through which it is asserting its effects by exploring alternative possible death pathways that are engaged upon Essiac® exposure. Likewise, it would be interesting to definitively uncover if Essiac® LHE can engage and activate specific ER subtypes, especially within various ER+ and ER- breast cancer cell lines.
Author contributions: J.R. and S.A.V conceived, designed, and collected the data and literature for the manuscript. J.R., I.J. and M.O. performed the experiments and assisted in the data analysis. S.A.V. supervised the study. J.R. and S.A.V. wrote the manuscript and all authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgments: We would like to acknowledge Agnieszka Witanis and Rachel Villareal, for their technical support during experimental set ups, and for being great lab partners. We would also like to thank Jessica M. Ochoa and Marissa E. Ochoa for their ideas and helpful discussions of this work.
Funding: This research received no external funding outside of Whittier College. Work conducted by J.R. and M.O. was supported by the Barbara Ondrasik ’57 and David Groce Fellowship for Undergraduate Research.
Conflicts of interest: Authors declare no conflict of interest with the authorship and or publication of this article.
- Complementary, alternative, or integrative health: What’s in a name? Available online: https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name. (accessed on 3 December 2020).
- WHO Global report on traditional and complementary medicine (2019) World health organization, Ed, World health organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151543-6.
- Saydah SH, Eberhardt MS (2006) Use of Complementary and alternative medicine among adults with chronic diseases: United states 2002. J Altern Complement Med 12: 805-812. [Crossref]
- Dy GK, Bekele L, Hanson LJ, Furth A, Mandrekar S, et al. (2004) Complementary and alternative medicine use by patients enrolled onto phase I clinical trials. J Clin Oncol 22: 4810-4815. [Crossref]
- Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE (2000) Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol 18: 2505-2514. [Crossref]
- Gansler T, Kaw C, Crammer C, Smith T (2008) A population-based study of prevalence of complementary methods use by cancer survivors. Cancer 113: 1048-1057. [Crossref]
- Swarup AB, Barrett W, Jazieh AR (2006) The use of complementary and alternative medicine by cancer patients undergoing radiation therapy. Am J Clin Oncol 29: 468-473. [Crossref]
- Gray RE, Greenberg M, Fitch M, Parry N, Douglas RN, et al. (1998) Perspectives of Cancer survivors interested in unconventional therapies. Journal of Psychosocial Oncology 15: 149-171.
- Boon H, Stewart M, Kennard MA, Gray R, Sawka C, et al. (2000) Use of complementary/alternative medicine by breast cancer survivors in Ontario: Prevalence and perceptions. J Clin Oncol 18: 2515-2521. [Crossref]
- Kaegi E and Institute (1998) Unconventional therapies for cancer: 1. Essiac. CMAJ 158: 897-902.
- Smith M, Pharms MR, Boon HS (1999) Counseling cancer patients about herbal medicine. Patient Educ Couns 12: 109-120. [Crossref]
- Essiac Handbook J. Percival 1999.Pdf.
- Tamayo C, Richardson MA, Diamond S, Skoda I (2000) The chemistry and biological activity of herbs used in Flor-Essence2 Herbal tonic and Essiac2. Phytother Res 14: 1-14. [Crossref]
- Leonard SS, Keil D, Mehlman T, Proper S, Shi X, et al. (2006) Essiac tea: Scavenging of reactive oxygen species and effects on DNA damage. J Ethnopharmacol 103: 288-296. [Crossref]
- Seely D, Kennedy DA, Myers SP, Cheras PA, Lin D, et al. (2007) In vitro analysis of the herbal compound Essiac®. Anticancer Research 27: 3875-3882. [Crossref]
- Cheung S, Lim KT, Tai J (2005) Antioxidant and anti-inflammatory properties of ESSIAC and Flor-essence. Oncol Rep 14: 1345-1350. [Crossref]
- Tai J, Cheung S, Wong S, Lowe C (2004) In vitro comparison of Essiac and flor-essence on human tumor cell lines. Oncol Rep 11: 471-476. [Crossref]
- Ottenweller J, Putt K, Blumenthal EJ, Dhawale S, Dhawale SW (2004) Inhibition of prostate cancer-cell proliferation by Essiac. J Altern Complement Med 10: 687-691. [Crossref]
- Kulp KS, Montgomery JL, Nelson DO, Cutter B, Latham ER, et al. (2006) Essiac® and Flor-Essence® herbal tonics stimulate the in vitro growth of human breast cancer cells. Breast Cancer Res Treat 98: 249-259. [Crossref]
- Eberding A, Madera C, Xie S, Wood CA, Brown PN, et al. (2007) Evaluation of the Antiproliferative effects of EssiacTM on in vitro and in vivo models of prostate cancer compared to paclitaxel. Nutr Cancer 58: 188-196. [Crossref]
- Zick SM, Sen A, Feng Y, Green J, Olatunde S, et al. (2006) Trial of Essiac to ascertain its effect in women with breast cancer (TEA-BC). J Altern Complement Med 12: 971-980. [Crossref]
- Riddle DL, Blumenthal T, Meyer BJ, Priess JR (1997) C. Elegans II. Eds, 2nd ed, Cold Spring harbor Laboratory press: Cold spring harbor (NY), ISBN 978-0-87969-532-3.
- Nicholas HR, Hodgkin J (2004) Responses to infection and possible recognition strategies in the innate immune system of caenorhabditis elegans. Mol Immunol 41: 479-493. [Crossref]
- Brys K, Vanfleteren JR, Braeckman BP (2007) Testing the rate-of-living/oxidative damage theory of aging in the nematode model caenorhabditis elegans. Exp Gerontol 42: 845-851. [Crossref]
- Ayuda-Durán B, González-Manzano S, González-Paramás AM, Santos-Buelga C (2020) Caenorhabditis Elegans as a model organism to evaluate the antioxidant effects of phytochemicals. Molecules 25: :3194. [Crossref]
- Senchuk MM, Dues DJ, Van Raamsdonk JM (2017) Measuring oxidative stress in caenorhabditis elegans: Paraquat and juglone sensitivity assays. Bio Protoc 7:e2086. [Crossref]
- Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB (1987) Evaluation of a tetrazolium-based semiautomated colorimetrie assay: Assessment of radiosensitivity. Cancer Res 47: 943-946. [Crossref]
- Barltrop JA, Owen TC, Cory AH, Cory JG (1991) 5-(3-Carboxymethoxyphenyl)-2-(4,5-Dimethylthiazolyl)-3-(4-Sulfophenyl)Tetrazolium, inner salt (MTS) and related analogs of 3-(4,5-Dimethylthiazolyl)-2,5-Diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans as cell-viability indicators. Bioorganic & Medicinal Chemistry Letters 1: 611-614.
- Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, et al. (1995) Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med 182: 1545-1556.
- Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH (2013) Caspase-9, Caspase-3 and Caspase-7 Have distinct roles during intrinsic apoptosis. BMC Cell Biol 14: 32. [Crossref]
- Aldossary SA (2019) Review on pharmacology of cisplatin: Clinical use, toxicity and mechanism of resistance of cisplatin. Biomedical and Pharmacology Journal 12: 7-15.
- Lai CH, Chou CY, Ch’ang LY, Liu CS, Lin W (2000) Identification of novel human genes evolutionarily conserved in caenorhabditis elegans by comparative proteomics. Genome Res 10: 703-713. [Crossref]
- Lawler DE, Chew YL, Hawk JD, Aljobeh A, Schafer WR, et al. (2019) Automated analysis of sleep in adult c. elegans with closed-loop assessment of state-dependent neural activity. bioRxiv 791764.
- Alexander AG, Marfil V, Li C (2014) Use of caenorhabditis elegans as a model to study alzheimer’s disease and other neurodegenerative diseases. Front Genet 5: 279. [Crossref]
- Ottenweller J, Putt K, Blumenthal EJ, Dhawale S, Dhawale SW (2004) Inhibition of prostate cancer-cell proliferation by Essiac®. J Altern Complement Med 10: 687-691. [Crossref]
- Mal R, Magner A, David J, Datta J, Vallabhaneni M, et al. (2020) Estrogen receptor beta (ERβ): A ligand activated tumor suppressor. Front Oncol 10: 587386. [Crossref]
- Lecomte S, Demay F, Ferrière F, Pakdel F (2017) Phytochemicals targeting estrogen receptors: Beneficial rather than adverse effects? Int J Mol Sci 18:1381. [Crossref]
- Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F (2014) Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 90: 13-29. [Crossref]
- Pan MH, Chiou YS, Chen LH, Ho CT (2015) Breast cancer chemoprevention by dietary natural phenolic compounds: Specific epigenetic related molecular targets. Mol Nutr Food Res 59: 21-35. [Crossref]
- Chi F, Wu R, Zeng YC, Xing R, Liu Y, et al. (2013) Post-diagnosis soy food intake and breast cancer survival: A meta-analysis of cohort studies. Asian Pac J Cancer Prev 14: 2407-2412. [Crossref]
- Uifălean A, Schneider S, Gierok P, Ionescu C, Iuga CA, et al. (2016) The impact of soy isoflavones on MCF-7 and MDA-MB-231 breast cancer cells using a global metabolomic approach. Int J Mol Sci 17: 1443. [Crossref]
- Oliveira CA, Nie R, Carnes K, Franca LR, Prins GS, et al. (2003) The antiestrogen ICI 182,780 decreases the expression of estrogen receptor-alpha but has no effect on estrogen receptor-beta and androgen receptor in rat efferent ductules. Reprod Biol Endocrinol 1: 75. [Crossref]








