Abstract
Developmental instability theory suggests that variation in some body parts in part can reflect the ability to buffer development from key environmental and genetic perturbations. Support for this approach comes mainly from assessment of fluctuating asymmetry, or deviations from symmetry of body features that are symmetric at the population level. In order to study dentalasymmetry in domestic goats, we sampled 22 adult goat skulls.Bucco-palatine and mesio-distal distances (width and length) of thesecond molar on each side for each skull, on their occlusal aspect, were measured and compared using standard lineal methods.There was evidence of directional asymmetry, that is, unilateral mastication habits, being the left teeth of the arch slightly but systematically longer than the right.This directional change supported a right chewing side preference in the sample. It is important to highlight that the sample comes from non-pathological specimens. Therefore, it does depict the sample population of animals usedin general.The observed asymmetries was not associated with any other cranio-facial abnormalities.
Key words
Capra, directional asymmetry, fluctuating asymmetry, matched symmetry, White Rasquera goat
Introduction
Animals body tends to present bilateral symmetric skeletal development, which implies that both right and left sideshave the same size and shape[1]. However, this is no more than a tendency, as asymmetry is commonly foundand reported inbiological literatures both at species, organ, tissue cellular and genetic levels and are not necessarily associated with syndromes, traumas, or pathologies[2].
Developmental stability(DS) is defined as the ability of organisms to withstand genetic or environmental disturbances during their development[3]. As DS reflects the capacity of organisms to producean optimum phenotype despite perturbations during development, its appraisal can be used to evaluate these stresses[3], whichmaybe environmental or genetic in origin[3]. For the former, a large number of stressor factors -food deprivation, temperature, pollution, and so on- have been shown to contribute significantly to the DI (developmental instability) of organisms[4].
But most of bilateral asymmetries are subtle and go unnoticed on casual clinical appraisal, so they require precise comparisons to be detected[5].Fluctuating asymmetry (FA) is frequently used to appraise DI[3].The idea behind this concept is that individuals oflowgenetic quality cannot control their development precisely, and consequently more often develop different phenotypes on both sides[6].Low levels of FA are then seen as indicators of overall quality or general health condition of individuals.Asymmetry of an individual is measured as the right minus the left value (L-R) of a bilaterally paired trait (homologous dimensions)[3,7], occurring when the sample distribution of the left-right differences is centered on zero[5].The small random deviations in FA result in a normal or leptokurtic distribution of asymmetry around a mean of zero[7].Although subtracting the measurement of the right side of a trait from that of the left side forms the basis of the analysis, accurately quantifying FA is not simple[7]. The measurement of FA is complicated by the distribution of measurement error (that component of the overall variance due to imprecision of measurements)[7]. Therefore, in order to establish that real differences in symmetry rather than just measurement error are being reported, it is imperative to establish that the measures of FA explain statistically significant proportions of the observed total variance between bothsides[7].
FA needs to be distinguished from two other types of asymmetry: directional asymmetry (DA) and antisymmetry (AS) [3,7], two conspicuously other asymmetrical forms in animals[8]. DA occurs when one side of a bilateral character is systematically larger/smallerthan the other, so the mean of the (L-R) normal distribution of the population is different from zero [3,5,9].On the other hand, a typicalantisymmetry (AS), which can be considered a macroscopic form of FA,trait leads to a bimodal (L-R) distribution centred on zero[3,9]. A prediction of AS is that the mean value of unsigned residuals from a linear regression of unsigned asymmetry against trait expression is subtle or absent as expression increases[10]. Prior assessment of DA andASmust be done instudies of symmetries, not only for the biological information it provides, but also to estimate if FA is potentially biased[1].
Mammalian molar teeth are designed to function by making species specific contacts with each other on the uppermaxillas and lower mandibles[11]. Molars cannot function without such occlusion[11], each tooth aligning precisely with its counterpart on the opposing jaw[11]. In the absence of any asymmetric constraints, tooth wearing on opposing jaws is coordinated, so there were only slight differences in shape between opposing teeth. Asymmetry between bilateral teeth in the dental arch in laboratory animals shows increased expression after exposure to external stress during development[12], but in humans, sometimes they are acquired, anexample is observed in chewing side preference or trauma.Few documented evidence existsin this regards on domestic mammals. Theaim of this investigation was to study bilateral asymmetry in teeth of domestic goats, and concretely of the second upper molars.
Materials and methods
Measurements and statistics
A sample of 22edentulousRasqueragoat skulls,representing animals of above 10 months of agewas studied. Maximal bucco-palatine distance (width)andmaximal mesio-distal distance(length)of secondupper molar(M2) foreach skull, for each side,on occlusal aspect, were measured two times in two temporally separated sessionsby the second author by using a digital-readout sliding caliperprecise to 0.01 mm.
As distribution of measurements (replicas pooled) appeared as being not-normally distributed for length ( W =0.952, p =0.002, assessed by a Shapiro-Wilk W test), non-parametric tests were performed for the analysis. Measurement error and differences between sides were analysed by a two-way-model NPMANOVA including all measurements as dependent variables, replicaand sideas factors, and Gower distances and 9,999 permutations.The error of the method was also determined through intraclass correlation coefficient (ICC) to assess intra-observer reliability. Then, averaged values were obtained, and a Wilcoxon W paired test was used for comparing signed left-right(L-R)matched values. A Kolmogorov-Smirnov D test was used for comparing overall equal distribution of both sides. Finally, a new Wilcoxon W paired test was applied to signed relative differences {(L-R)/(L+R)}to know their departure from 0 value.
All statistics were performed using the PAST software[13]. Confidence level was established at 0.05.
Results
With mean squares values clearly below values for sides(Table 1). In other words, measurements for both populations were shown to be highly repeatable indicating a very low influence of error on measurements. Intra-observer ICCwas 0.96 for the evaluated measurement, thus reinforcing reliability of two replicas, with an average difference between observations of 0.28 mm. Signed right-left (L-R) side measurements appeared also not statistically significant both for width as for length(Table 1). Average for each measurement and side wereobtained(Table 2).Kolmogorov-Smirnov test demonstrated different overall distributions for both measurements ( D =0.454, p =0.013). They showed (L-R)statistical differences for length ( W =198, p =0.018) but not for width ( W =170, p =0.163) in the Wilcoxon W paired test.A new Wilcoxon test for signed relative differences {(L-R)/(L+R)} corroborated differences for length ( W =199, p =0.017) but not for width ( W =169, p =0.176). Signed relative differences for length presented a positive sign (median of 0.005)(Figure 1).
Table 1.Two-way-model NPMANOVA including all measurements as dependent variables, and replica (R) and side (S) as factors, to detect error and differences between sides, for M2 length and width (n=22). The between-replicas variation for all teeth was not statistically significative. Right-left side measurements appeared also not statistically significative, either.
Length |
Sum of squares |
Degrees of
freedom |
Mean
squares |
F |
P |
R |
2.52E-05 |
1 |
2.52E-05 |
0.001 |
0.981 |
S |
0.022338 |
1 |
0.022338 |
0.483 |
0.500 |
Interaction |
0.000107 |
1 |
0.000107 |
0.002 |
0.960 |
Width |
|
|
|
|
|
R |
0.005891 |
1 |
0.005891 |
0.123 |
0.734 |
S |
0.007898 |
1 |
0.007898 |
0.164 |
0.694 |
Interaction |
0.001133 |
1 |
0.001133 |
0.024 |
0.878 |
Table 2. Main descriptive statistics for length and width of second upper molar (n=22). Linear measurements in mm. Kolmogorov-Smirnov test demonstrated different overall distributions for both measurements ( D =0.454, p =0.013). They presented (L-R) statistical differences for length ( W =198, p =0.018) but not for width ( W =170, p =0.163) in the Wilcoxon W paired test.
| |
Length left |
Left width |
Length right |
Width right |
Minimal value |
12.5 |
11.0 |
12.4 |
10.6 |
Maximal value |
18.9 |
15.1 |
19.2 |
15.3 |
Mean |
15.4 |
13.0 |
15.2 |
13.1 |
Standard deviation |
1.410 |
0.904 |
1.474 |
1.142 |
Median |
15.0 |
13.0 |
14.8 |
13.3 |
Skewness |
0.597 |
-0.107 |
0.891 |
-0.318 |
Kurtosis |
0.472 |
0.184 |
0.945 |
-0.266 |
Geometric mean |
15.3 |
13.0 |
15.1 |
13.0 |
Coefficient of variation (%) |
9.1 |
6.9 |
9.6 |
8.7 |
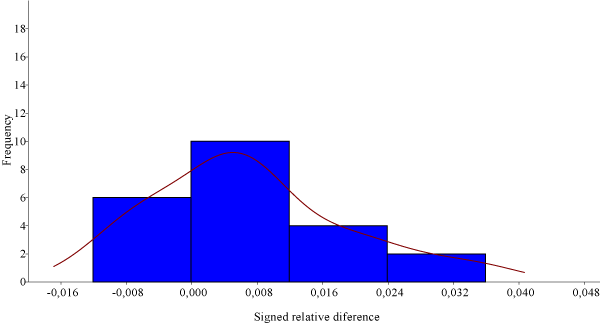
Figure 1.Histogram for signed relative differences {(L-R)/(L+R)} of M2 lengths (n=22 pairs). Data presented a positive sign (median of 0.005). The asymmetry distribution made for each of the two dimensions demonstrated that the measurements of length explicitly dominates on the left side.
Discussion
The two sides of the mammalianbody are assumed to have similargeneticinformation[5]. During development many environmental issues may cause FA, such as side differences in times of primary tooth exfoliation and or germination, theposition and orientation of the developing successor’s tooth buds, differencessuch asseen in eruptive tempos and pathways, differences in tooth emergence and sequence, positions of antagonists [5].None of these seem to have any effects on the present investigation since there were no detected FA inM2 in goats.
This study detectedDA inthe left M2 length tending to exceed the right . Detected M2 asymmetries did not present association with apparent pathologies, denoting a normal rostro-caudal masticatory force different for both sides . The wear process on the surface of teeth is a complex phenomenonthat dependsondiettype of individuals, captivity[14]and breed variation in the hardness of dental tissues, the ingestion of hard phytoliths (grit) in the dietinfluence the pattern, force and the direction of the chewing actions.The sizes, shapes, and angles of the opposing occlusal surfaces, and the relationship of cusps and crest patterns to the occlusal motion of cheek teeth contribute to tooth wear mappings[15]. Goats survive on coarse grass,the ingestion of large quantities of coarse foodstuffs containing much abrasive silicatespredisposes to dental abrasion due to force demands on the teeth. Side preferences in chewing motion may also be due to innervation differences between both facial segments due to injuries or difficult access to preferred food choice[16]. So, if there are unbalanced attritive (wear) forces due to a chewing side preference, and if animals tend to chew the hard food mostly with their left side and soft food with both left and right[17], teeth wearing will be exacerbated, although not enough to cause pathological disharmonies between masticatory right and left sides.
In conclusion, we think that obtained DA length is explained by a differential masticatory use, that is, unilateral mastication habits, in thebelieving that molar FA would reflect environmental stress and variability while DA would reflect an asymmetrical biomechanical loading.
It has been suggested that some ungulates are one-sided chewers, a condition that could significantly affect attrition patterns[18,19,20,21], but none have measured bilateral teeth variables on goats, so for comparative purposes,data are inexistent. Moreover, further studies should be conducted to better comprehend the factors that could be related to skeletal asymmetries, as well as to attempt to determine the weight of genetics as an etiological factor of such alterations.
Acknowledgement
Authors wish to acknowledge the help of Josué Sabaté for allowing the study of his private collection of White Rasquera skulls. The rest of skulls are stored in the bone collection of the Department of Animals Science of the University of Lleida, Catalonia, Spain.
References
- Briones C, Guiñez R (2008) Una revisión de la asimetría bilateral en bivalvos. Revista de Biología Marina y Oceanografía 43: 1-6.
- Klingenberg CP (2015) Analyzing Fluctuating Asymmetry with Geometric Morphometrics: Concepts, Methods, and Applications. Symmetry 7: 843-934.
- Auffray JC, Debat V, Alibert P (1999) Shape asymmetry and developmental stability. In Mark JCM, Chaplain AJ, Singh GD, Ed. On growth and form: spatio-temporal pattern formation in biology. John Wiley and Sons Ltd 309-324.
- Gilbert SF (2010) Developmental biology, Massachussettes: Sinauer Associates Inc. Sunderland.
- Harris EF, Bodford K (2007) Bilateral asymmetry in the tooth relationships of orthodontic patients. Angle Orthod 77: 779-786. [Crossref]
- Galeotti P, Sacchi R, Vicario V (2005) Fluctuating asymmetry in body traits increases predation risks: tawny owl selection against asymmetric woodmice. Evolutionary Ecology 19: 405-418.
- Tomkins JL, Andrews S (2001) Fluctuating Asymmetry. Encyclopedia of Life Sciences 1-5.
- Palmer AR (1996) From symmetry to asymmetry: phylogenetic patterns of asymmetry variation in animals and their evolutionary significance. Proc Natl Acad Sci USA 93:14279-14286. [Crossref]
- Lens L, Van Dongen S, Kark S, Matthysen E (2002) Fluctuating asymmetry as an indicator of fitness: can we bridge the gap between studies? Biol Rev Camb Philos Soc 77: 27-38. [Crossref]
- Rowe L, Repasky RR, Palmer AR (1997) Size-dependent asymmetry: fluctuating asymmetry versus antisymmetry and its relevance to condition-dependent signaling. Evolution 51: 1401-1408. [Crossref]
- McCollum M, Sharpe PT (2001) Evolution and development of teeth. J Anat 199: 153-159. [Crossref]
- Pirttiniemi P, Alvesalo L, Silvén O, Heikkilä J, Julku J, et al. (1998) Asymmetry in the occlusal morphology of first permanent molars in 45, X/46,XX mosaics. Arch Oral Biol 43: 25-32. [Crossref]
- Hammer Ø, Harper DAT, Ryan PD (2001) PAST v. 2.17c. Palaeontologia Electronica, 4:1-229.
- Clauss M, Franz-Odendaal TA, Brasch J, Castell JC, Kaiser T (2007) Tothwear in Captive Giraffe (Giraffa camellopardalis): Mesowear analysis classifies free-ranging specimens as browsers but captive ones as grazers. J Zoo Wildl Med 38: 433-445. [Crossref]
- Dixon PM (2002) The gross, histological, and ultrastructural anatomy of equine teeth and their relationship to disease. In AAEP Proceedings. AAEP 421-437.
- Olopade JO, Onwuka SK (2005) Some Aspects of the clinical Anatomy of the mandibular and maxillofacial Regions of the West African Dwarf Goat in Nigeria. Int J Morphol 23: 33-38.
- Dias GJ, Cook RB, Mirhosseini M (2011) Influence of food consistency on growth and morphology of the mandibular condyle. Clinical Anatomy 24: 590-598. [Crossref]
- Parés-Casanova PM (2013) Allometric shape variation in Ovis aries mandibles: A digital morphometric analysis. Journal of Morphological Sciences 30.
- Parés-Casanova PM (2014a) Existence of mandibular directional asymmetry in the European wild boar (Sus scrofa linnaeus, 1758). Journal of Morphological Sciences 31.
- Parés-Casanova PM (2014b) Existence of mandibular directional asymmetry in the European wild boar (Sus scrofa Linnaeus, 1758). J Morphol Sci 31:1-5.
- Parés-Casanova PM (2014c) Size asymmetries in equine upper molar series. ECORFAN Journal 5: 2055-2069.

