Abstract
Introduction: Previously, we reported that nutritional supplementation with myo-inositol in growing mice specifically augments mandibular endochondral growth. However, details of chondrocytic differentiation induced by myo-inositol is still remain unclear.
Methods: The chondrocyte cell line, ATDC5 was used in this study for cell culture experiments. Cells were cultured in the presence or absence of myo-inositol or recombinant BMP4 (100 ng/mL. After cultivation, RNA were extracted from the cells, were reverse transcribed (RT), and the expression of chondrocytic differentiation markers were measured by realtime PCR. To further analyze the effects of these molecules on chondrocytic differentiation, the protein level expression of type X collagen (Col X) were examined by immuno-fluorescent analysis.
Results: Realtime RT-PCR analysis clearly demonstrated that chondrocytic differentiation markers, such as collagen type II, Col X, and SOX9, were augmented by BMP4, as positive control. Interestingly, myo-inositol were also augmented these chondrocytic differentiation markers. Immuno-fluorescent analysis revealed that not only BMP4 but also myo-inositol increased the expression of Col X.
Conclusion: We discovered that myo-inositol augments chondrocytic differentiation in ATDC5. Our results suggest that myo-inositol would be beneficial supplement for not only augmentation of mandibular growth, but also for maintaining favorable cartilage homeostasis.
Keywords
chondrocyte, differentiation, Col II, Col X, Pik3cd, myo-inositol
Abbreviations
RT: reverse transcribed; Col X: type X collagen; Col II: type II collagen; RPS18: ribosomal protein S18
Introduction
Mandibular retrognathism can occur due to either a developmental abnormality or an unfavorable positioning of the developing jaws [1,2]. Mandibular retrognathism can lead to several problems, such as respiratory difficulties, temporomandibular joint disorders, reduced chewing function, and aesthetic issues related to maxillofacial deformities [3-6]. Currently, orthodontic treatment for skeletal mandibular retroversion in children during the growth and development period is generally based on the use of functional orthognathic appliances to promote anterior mandibular growth.
The most common method for orthodontic treatment of skeletal mandibular retrognathism in growing children is to promote forward growth of the mandible using functional orthognathic appliances, such as activator and bionator [7-9]. However, the long-term effect of this type of orthognathic treatment is not stable, and a new treatment method with high predictability is desired [10].
We have recently reported that myo-inositol supplementation for the mouse diet can promote mandibular-specific growth [11]. In the report, we discovered that Pik3cd, an enzyme involved in myo-inositol metabolism, is specifically up-regulated in mandibular chondylar cartilage, and myo-inositol supplementation augments cell proliferation and chondrocytic differentiation. However, details of chondrocytic differentiation induced by myo-inositol is still remain unclear [12].
Functions of Pik3cd in cells were extensively explored. Constitutive PI3K activation is the result of autocrine IGF-1/IGF-1R signaling in 70% of acute myeloid leukemia [13]. PI3K pathway defects lead to immunodeficiency and immune dysregulation [14]. Furthermore, Pik3cd plays a role in maintaining favorable immune responses [15,16].
Chondrocytic differentiation is regulated by several axes, such as transcription factor SOX9, hedgehog signaling, Fibroblast growth factors, cell-matrix interactions including N-cadherin and integrins, and epigenetic mechanisms [17-23]. To our knowledge, there is no report on the relationship between Pik3cd and chondrocytic differentiation.
In this report, we explored the phenomenon of myo-inositol-induced chondrocytic differentiation using mouse chondrocytic cell-line, ATDC5.
Materials and methods
Chemicals
Myo-inositol was purchased from Wako Pure Chemical Industries, Ltd., (Osaka, Japan).
Cells and Cell Culture
The chondrocyte cell line, ATDC5 was obtained from Riken Bioresource Center (Tsukuba, Japan). ATDC5 was cultured in DMEM/Ham's F-12 with L-Gln and Sodium Pyruvate (FUJIFILM WakoChemicals, Tokyo, Japan), without HEPES, containing 10% fetal bovine serum and supplemented with antibiotics (100 U/mL of penicillin and 100 mg/mL of streptomycin).
Cells were cultured at 37°C in a 5% CO2 incubator. Cells were cultured in the presence or absence of myo-inositol (100 µM). In some experiments, cells were stimulated with BMP4 (100 ng/mL: FUJIFILM Wako Chemicals, Tokyo, Japan) to induce chondrocytic differentiation.
RNA extraction and Reverse Transcription (RT)
RNA from cultured cells were extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. After measuring the RNA concentration, equal amounts of RNA (500 ng) were reverse transcribed using iScript cDNA Supermix (Bio-Rad Laboratories, Hercules, CA). cDNA was diluted five-fold with Tris-EDTA buffer and used for subsequent Real-time RT-PCR analysis.
Real-time RT-PCR analysis
Real-time RT-PCR was performed using SsoFast EvaGreen-Supermix (Bio-Rad, Laboratories) on a CFX connect Real-Time PCR System (Bio-Rad Laboratories). Fold changes of genes of interest were calculated by using the -ΔΔCT method with ribosomal protein S18 (RPS18) as a reference gene. Primer sequences for mouse collagen type II (Col II), mouse collagen type X (Col X), SOX9, and RPS18 were as follows:
Col II:
(Forward) 5′-GGGAATGTCCTCTGCGATGAC-3’,
(Reverse) 5′-GAAGGGGATCTCGGGGTTG-3’
Col X:
(Forward) 5′-TTCTGCTGCTAATGTTCTTGACC-3’,
(Reverse) 5′-GGGATGAAGTATTGTGTCTTGGG-3’
SOX9:
(Forward) 5′-AGTACCCGCATCTGCACAAC-3’,
(Forward) 5′-ACGAAGGGTCTCTTCTCGCT-3’
RPS18:
(Forward) 5′-AGTTCCAGCACATTTTGCGAG-3’,
(Reverse) 5′-TCATCCTCCGTGAGTTCTCCA-3’
Immunofluorescent staining
Cells were seeded into 6-well plates containing glass coverslips (Matsunami Glass Co.,Ltd, Osaka, Japan). After cells got confluent, culture medium was changed to the following condition; control medium, medium containing myo-inositol (final 100 mM), medium containing BMP4 (100 ng/mL: FUJIFILM WakoChemicals, Tokyo, Japan), and further cultured for 7 days. Cells were then fixed with ice-cold methanol for 15 minutes, were washed with PBS-T at 3 times, and were blocked in 10% BSA in PBS for 1 hour at room temperature. Cells were then incubated with anti-Col10A1 Ab (1:100) (Cloud-clone corp. Wuhan, China) in Can get signal solution (Toyobo, Osaka, Japan), washed with PBS-T, incubated with Alexa Fluor 488–conjugated secondary Ab (1:1000) (Abcam, Cambridge, MA), and washed again with PBS-T. Nuclei were stained with DAPI (1µg/ml)(Sigma-Aldrich Co., St Louis, MO)) and fluorescent photographs were taken with a BZ-9000 microscope (Keyence, Osaka, Japan), with same exposure condition.
The percent of green fluorescence positive area per field were calculated by using ImageJ software (National Institutes of Health, Bethesda, MD) from at least 19 images in each culture condition.
Statistical analysis
All data are presented as the mean ± standard error. Multiple comparisons were performed using Tukey’s test. A p < 0.05 was considered statistically significant.
Results
Markers for chondrocytic differentiation were augmented by myo-inositol in mRNA level
Realtime RT-PCR analysis revealed that Myo-inositol augmented the expression of markers for chondrocytic differentiation at mRNA level (Figure 1). Among them, Sox9, which is known as a critical factor for chondrocyte differentiation, was augmented by myo-inositol at similar extent to BMP4 stimulation at day-3 [24,25]. Col II expression at day-3 was augmented by myo-inositol, though the induction was higher in the BMP4 treatment. Interestingly, expression of Col X, which is known as terminal differentiation marker for chondrocytic differentiation, was stable at day-3, though the expression was significantly induced at day-5 [26].
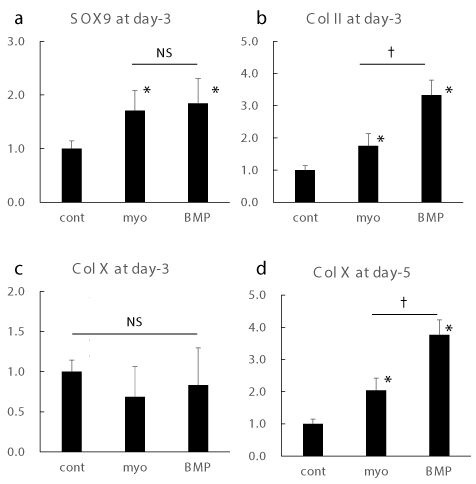
Figure 1. Realtime RT-PCR for chondrocytic differentiation markers
Gene expressions of sox9 (a), Col II (b), and Col X (c) at day 3, and Col X expression at day 5 (d) were shown. *: p < 0.05 versus control. †: p < 0.05 between groups.
These data suggest that myo-inositol augment the mRNA expression of chondrocytic differentiation markers similar to BMP4.
Myo-inositol augmented Col X protein expression
Then we examined protein level expression of Col X (Figure 2). The expression of Col X at day 7 was increased by BMP4 (Figure 2c). Not only BMP4, but also myo-inositol augmented Col X expression (Figure 2b). Percent of immuno-positive area in the field were calculated using 19 to 22 photographs in each group (Figure 2d). Compared to control, myo-inositol and BMP4 augmented Col X positive area. Col X positive area in BMP4 group were statistically higher than that in myo-inositol group.
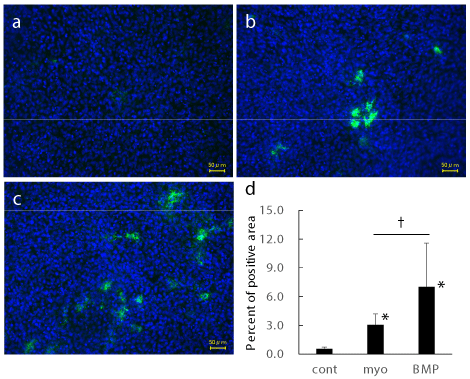
Figure 2. Immunofluorescent analysis for Col X
Representative photographs of control (a), myo-inositol (b), and BMP4 (c) groups were shown.
Percent of positive area from 19 to 22 photographs in each group were shown. *: p < 0.05 versus control. †: p < 0.05 between groups.
These data suggest that myo-inositol augment protein level expression of chondrocytic differentiation markers similar to BMP4.
Discussion
In this study, we firstly discovered that myo-inositol augments chondrocytic differentiation in ATDC5. Our results suggest that myo-inositol would be beneficial supplement for not only augmentation of mandibular growth, but also for maintaining favorable cartilage homeostasis.
We used ATDC5 as pre-chondrocyte in this experiments, because ATDC5 expresses Pik3CD similar to mandibular condylar cartilage [11]. In the previous report, we demonstrated that Pik3CD-expressing cells such as mandibular condylar cartilage and ATDC5 exhibited augmentation of cell proliferation in the Pik3CD-dependent manner revealed by the use of Pik3CD inhibitor. Pik3cd, which is one of the phosphatidylinositol 3-kinase, consists of family, and these family enzymes act as key enzymes to produce phosphatidylinositol, a second messenger for intracellular signaling, including cell proliferation [12,27]. Taken these informations together with our results, myo-inositol would induce intracellular signaling via Pik3cd, and augment chondrocytic differentiation. Therefore, another cartilage that express less Pik3CD would be little response to myo-inositol. Further experiments are necessary to clarify the issue.
Myo-inositol augmented Sox9, which is known as a critical factor for chondrocyte differentiation, at similar extent to BMP4 stimulation [28,29]. This indicates myo-inositol would be beneficial for cartilage differentiation. Indeed, our results clearly demonstrated that another chondrocyte differentiation markers were also augmented by myo-inositol. Chondrocytic differentiation is regulated by several axes. Transcription factor, SOX9 is thought to be the master regulator for chondrogenesis [17]. Hedgehog signaling is reported to play a key role in cartilage formation [18]. Exploration for genetic mutation revealed that fibroblast growth factors play a role in cartilage formation [19]. On the other hand, cell microenvironment by cell-matrix interactions including N-cadherin and integrins regulate cartilage formation [20,21]. Though our data clearly demonstrated that myo-inositol augmented chondrocytic differentiation, the mechanism how myo-inositol interact with these regulatory axes in chondrocytic differentiation still remain undiscovered. Further experiments are also necessary to clarify the issue.
In this study, we firstly discovered that myo-inositol augments chondrocytic differentiation in ATDC5. Our results suggest that myo-inositol would be beneficial supplement for not only augmentation of mandibular growth, but also for maintaining favorable cartilage homeostasis.
Funding information
This research was supported in part by the following Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science; 22K10256, 22K10257, 22K10281, 21K10197, 21K10199, and 21K21026.
Competing interest
The authors declare that they have no competing interests.
References
- Egermark-Eriksson I, Ingervall B, Carlsson GE (1983) The dependence of mandibular dysfunction in children on functional and morphologic malocclusion. Am J Orthod 83: 187-194. [Crossref]
- Joshi N, Hamdan AM, Fakhouri WD (2014) Skeletal malocclusion: A developmental disorder with a life-long morbidity. J Clin Med Res 6: 399-408. [Crossref]
- Hamada T, Ono T, Otsuka R, Honda E, Harada K, et al. (2007) Mandibular distraction osteogenesis in a skeletal class ii patient with obstructive sleep apnea. Am J Orthod Dentofacial Orthop 131: 415-425. [Crossref]
- Henrikson T, Ekberg EC, Nilner M (1997) Symptoms and signs of temporomandibular disorders in girls with normal occlusion and class ii malocclusion. Acta Odontol Scand 55: 229-235. [Crossref]
- Kjellberg H (1995) Juvenile chronic arthritis. Dentofacial morphology, growth, mandibular function and orthodontic treatment. Swed Dent J Suppl 109: 1-56. [Crossref]
- Shelly AD, Southard TE, Southard KA, Casko JS, Jakobsen JR, et al. (2000) Evaluation of profile esthetic change with mandibular advancement surgery. Am J Orthod Dentofacial Orthop 117: 630-637. [Crossref]
- Jena AK, Duggal R, Parkash H (2006) Skeletal and dentoalveolar effects of twin-block and bionator appliances in the treatment of class ii malocclusion: A comparative study. Am J Orthod Dentofacial Orthop 130: 594-602. [Crossref]
- Xie J, Huang C, Yin K, Park J, Xu Y (2021) Effects of orthodontic treatment with activator appliance on patients with skeletal class ii malocclusion: A systematic review and meta-analysis. Ann Palliat Med 10: 12319-12334. [Crossref]
- Santamaría-Villegas A, Manrique-Hernandez R, Alvarez-Varela E, Restrepo-Serna C (2017) Effect of removable functional appliances on mandibular length in patients with class ii with retrognathism: Systematic review and meta-analysis. BMC Oral Health 17: 52. [Crossref]
- Cozza P, Baccetti T, Franchi L, De Toffol L, McNamara JA Jr (2006) Mandibular changes produced by functional appliances in class ii malocclusion: A systematic review. Am J Orthod Dentofacial Orthop 129: 599 [Crossref]
- Yamaguchi Y, Kanzaki H, Miyamoto Y, Itohiya K, Fukaya S, et al. (2019) Nutritional supplementation with myo-inositol in growing mice specifically augments mandibular endochondral growth. Bone 121: 181-190. [Crossref]
- Clayton E, McAdam S, Coadwell J, Chantry D, Turner M (2001) Structural organization of the mouse phosphatidylinositol 3-kinase p110d gene. Biochem Biophys Res Commun 280: 1328-1332. [Crossref]
- Park S, Chapuis N, Tamburini J, Bardet V, Cornillet-Lefebvre P, et al. (2010) Role of the pi3k/akt and mtor signaling pathways in acute myeloid leukemia. Haematologica 95: 819-828. [Crossref]
- Nunes-Santos CJ, Uzel G, Rosenzweig SD (2019) Pi3k pathway defects leading to immunodeficiency and immune dysregulation. J Allergy Clin Immunol 143: 1676-1687. [Crossref]
- Hebeis BJ, Vigorito E, Turner M (2004) The p110delta subunit of phosphoinositide 3-kinase is required for the lipopolysaccharide response of mouse b cells. Biochem Soc Trans 32: 789-791. [Crossref]
- Okkenhaug K, Bilancio A, Emery JL, Vanhaesebroeck B (2004) Phosphoinositide 3-kinase in t cell activation and survival. Biochem Soc Trans 32: 332-335. [Crossref]
- Hata K, Takahata Y, Murakami T, Nishimura R (2017) Transcriptional network controlling endochondral ossification J Bone Metab 24: 75-82. [Crossref]
- Ohba S (2016) Hedgehog signaling in endochondral ossification. J Dev Biol 4(2). [Crossref]
- Ornitz DM (2005) FGF signaling in the developing endochondral skeleton. Cytokine Growth Factor Rev 16: 205-213. [Crossref]
- Tavella S, Raffo P, Tacchetti C, Cancedda R, Castagnola P (1994) N-cam and n-cadherin expression during in vitro chondrogenesis. Exp Cell Res 215: 354-362. [Crossref]
- Loeser RF (2000) Chondrocyte integrin expression and function. Biorheology 37: 109-116.
- Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, et al. (2004) Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell 119: 555-566. [Crossref]
- Kozhemyakina E, Cohen T, Yao TP, Lassar AB (2009) Parathyroid hormone-related peptide represses chondrocyte hypertrophy through a protein phosphatase 2a/histone deacetylase 4/mef2 pathway. Mol Cell Biol 29: 5751-5762. [Crossref]
- Bi W, Deng JM, Zhang Z, Behringer RR, De Crombrugghe B (1999) Sox9 is required for cartilage formation. Nat Genet 22: 85-89. [Crossref]
- Akiyama H, Chaboissier M-C, Martin JF, Schedl A, De Crombrugghe B (2002) The transcription factor sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of sox5 and sox6. Genes Dev 16: 2813-2828. [Crossref]
- Harrington EK, Coon DJ, Kern MF, Svoboda KK (2010) Pth stimulated growth and decreased col-x deposition are phosphotidylinositol-3,4,5 triphosphate kinase and mitogen activating protein kinase dependent in avian sterna. Anat Rec (Hoboken) 293: 225-234. [Crossref]
- Bozulic L, Hemmings BA (2009) Pikking on pkb: Regulation of pkb activity by phosphorylation. Curr Opin Cell Biol 21: 256-261. [Crossref]
- Hino K, Saito A, Kido M, Kanemoto S, Asada R, et al. (2014) Master regulator for chondrogenesis, sox9, regulates transcriptional activation of the endoplasmic reticulum stress transducer bbf2h7/creb3l2 in chondrocytes. J Biol Chem 289: 13810-13820. [Crossref]
- Nishimura R, Hata K, Matsubara T, Wakabayashi M, Yoneda T (2012) Regulation of bone and cartilage development by network between bmp signalling and transcription factors. J Biochem 151: 247-254. [Crossref]


