Smoking and tobacco use continue to be the largest preventable causes of death. Although there are current pharmaceutical and behavioural therapies, the one-year sustained quit rate of these therapies is only 20-25% at best. Recently, an alternative biotherapeutic strategy has been proposed: enzymatic degradation of nicotine in the bloodstream preventing accumulation in the brain. The bacterial enzyme NicA2 oxidizes nicotine into pseudo-oxynicotine, a non-addictive compound already found in smokers. Proof-of-concept animal studies have shown that NicA2 can rapidly reduce the levels of nicotine accumulating in the brain after intravenous nicotine dosing, and NicA2 has shown to have efficacy in a continuous nicotine access self-administration rat model.
Enzymatic elimination of nicotine upon smoke inhalation to combat tobacco addiction is an innovative therapeutic concept. However, it is in line with recent clinical studies demonstrating that reduction in nicotine content in cigarettes (to 2.5% of normal levels) lead to significant reduction in the number of cigarettes smoked and higher smoking cessation rates compared to a control group smoking normal nicotine level cigarettes. Enzymatic degradation of nicotine appears to be more potent than nicotine-specific antibodies or vaccines for reducing nicotine distribution to brain, and if this proves to be the case in humans, it could also be more effective for enhancing smoking cessation rates and succeed where nicotine vaccines have failed thus far. The work reviewed in this article constitutes promising initial steps towards an urgently needed new effective treatment strategy in smoking cessation therapy.
Smoking and tobacco use continue to be the largest preventable causes of death [1]. In 2015, approximately 6.4 million deaths were attributed to smoking worldwide. Although most smokers are aware of the health risks, smoking cessation is usually difficult to maintain. Current pharmacological therapies for smoking cessation combined with counselling have significant clinical effects compared to counselling alone [2]. However, only 20-25% of smokers remain abstinent for at least 1 year after treatment [3]. This fact means that new, more efficacious drugs need to be developed.
Multiple meta-analyses have been conducted to investigate the pharmaceutical interventions for smoking cessation, and guidelines have been published by many organizations [2,4]. The first-line pharmacological therapy for smoking cessation are nicotine replacement products (patches, gums, inhalers, nasal sprays, tablets, and oral sprays). It evokes its effects by stimulating the nicotinic receptors in the ventral tegmental area of the brain releasing dopamine in the nucleus accumbens [5]. NRT can lead to a reduction in withdrawal symptoms in smokers who would like to quit. Varenicline works as a partial agonist of the nACh receptor also releasing dopamine [6]. Furthermore, Bupropion, a tricyclic antidepressant, can be used in smoking cessation therapy. It inhibits reuptake of dopamine, noradrenaline, and serotonin in the central nervous system, and is a non-competitive nicotine receptor antagonist. The inhibition of the levels of dopamine and noradrenalin are thought to be important for Bupropion to have its antismoking actions [7]. Varenicline and bupropion are usually prescribed and when used for 2-3 months achieve a doubling of the quit rate compared to placebo [8]. Furthermore, counselling should be given to help in smoking cessation. Brief advice alone given by a general practitioner result in a 2-3% increase in quit rates [9]. To stop smoking is to break a complex habit and addiction and, to achieve reasonable quit rates, it is necessary to provide psychological support combined with pharmacological drugs. However, even with optimal pharmacological therapies only 20–25% of smokers remain abstinent for at least 1 year after treatment. This means that new therapies need to be developed.
As an alternative to small-molecule-based therapies, immunotherapeutic approaches to smoking cessation and vaccination against nicotine were investigated in the last three decades [10]. Researchers showed that it is possible to link or conjugate psychoactive drugs (such as cocaine, heroin or nicotine) to carrier proteins, thus making these small molecules antigenic. This work led to the hypothesis that it may be possible to develop vaccines which can prevent or treat addiction to these drugs. The proposed mechanism of action is that vaccine-elicited antibodies target and capture the drug in the periphery, reducing the concentrations reaching the brain and reducing its reinforcing effects. Nicotine conjugate vaccines showed early promise preclinically but failed to demonstrate broad clinical efficacy in large clinical randomised controlled trials [10,11]. Although increased efficacy was observed in those individuals who attained the highest anti-nicotine antibody titres [10-12], indicating that an antibody-mediated strategy in smoking cessation could work, the levels of antibodies generally were too low and too variable to have a clinically relevant outcome [13]. Essentially, the challenge has been a lack of potency to alter the pharmacokinetics of nicotine sufficiently in order to eliminate its reinforcing effects across a broad population of smokers.
Recently, an alternative biotherapeutic strategy has been proposed: nicotine degradation via an enzymatic approach, eliminating its exposure to the brain [14]. Pseudomonas putida S16 is an example of a nicotine-degrading bacterial strain that can use nicotine as its nitrogen and carbon source. It was originally isolated from a field underneath continuous tobacco cropping in China and is able to metabolise nicotine to fumaric acid [15]. The enzyme found in the first committed step of S16’s nicotine degradation is NicA2, an amine oxidase. NicA2 oxidises nicotine to N-methylmyosmine, which undergoes rapid, spontaneous hydrolysis to pseudooxynicotine, a non-addictive compound already found in smokers.
Xue and colleagues studied the features of NicA2 in vitro to evaluate its potential as a starting point for the development of a nicotine-degrading drug for use in smoking cessation therapies [14]. They demonstrated that NicA2 has favourable characteristics such as high stability in buffer and mouse serum, as well as high catalytic activity at nicotine concentrations typically found in smokers’ blood [14].
NicA2 was subsequently evaluated in vivo through single-dose nicotine pharmacokinetic (PK) studies in rats pre-treated with a range of NicA2 doses [16,17]. Reduction in nicotine blood and brain levels was measured 1, 3 and 5 minutes after an intravenous bolus dose of 0.03 mg/kg nicotine. This nicotine dose is equivalent to 2 cigarettes with regard to milligrams of nicotine per kilogram of body weight. Short intervals were used, as the enzyme’s effectiveness is expected to be dependent on the rapid elimination of nicotine. While smokers achieve maximum levels of brain nicotine in 3 to 5 minutes, nicotine is initially detected in the brain 7 seconds after the first inhalation [18]. NicA2’s effects on nicotine distribution to the blood and brain were dependent on dose and time, as shown in the Figure 1 below [19]. When dosed at 5 mg/kg, blood levels of nicotine dropped to below the limit of quantitation of the assay (2 ng/ml), virtually eliminating nicotine from the bloodstream within 1 minute as compared to the control group. The levels of nicotine in the brain were also assessed, with a 10-mg/kg NicA2 dose lowering brain nicotine levels by 95% at 3 and 5 minutes after nicotine dosing as compared to the control group, while a higher dose of 20 mg/kg was needed for reducing brain nicotine levels to the same extent within one minute. As one minute is a practical time limit to euthanise the rats and to collect blood and brain samples, the onset of enzyme activity was evaluated in blood samples in vitro, where typical maximum blood levels of nicotine were degraded to below the level of detection within 10 seconds [17].
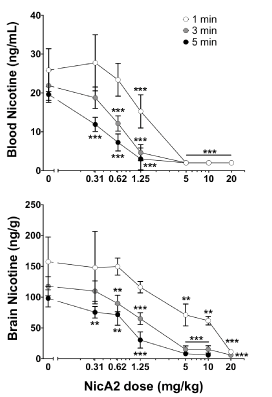
Figure 1. Reduction of blood and brain nicotine concentrations by NicA2. Rats were pre-treated with NicA2 IV and 5 minutes later received 0.03 mg/kg nicotine IV. Groups of eight rats had nicotine levels measured at 1, 3 or 5 minutes in blood (upper panel) and brain tissue (lower panel). Nicotine concentrations were rapidly reduced by NicA2 in a dose- and time-related manner. **p < 0.01, ***p < 0.001 compared to BSA using Bonferroni-corrected Welch’s t-tests. Reproduced with permission from [18].
In repeated nicotine dose experiments that simulated very heavy smoking, 5 doses of 0.03 mg/kg nicotine spaced 10 minutes apart (equivalent to 10 cigarettes over 40 minutes) were given intravenously to rats pre-treated with 10 mg/kg NicA2. Brain nicotine levels were lowered by the same degree after the 5th dose as after the 1st dose of nicotine, a potency never observed for immunotherapeutic approaches [17,19,20].
In order to enable longer-term in vivo testing, two different constructs fusing NicA2 to an albumin-binding domain (NicA2-ABD) [21] have been independently reported [17,22]. Circulating half-life was extended from a few hours to 2.5 days in rats, similar to that of endogenous serum albumin, without affecting its catalytic activity [16,17]. Consistent with the effects of NicA2 on reduced nicotine distribution in the brain, when such an enzyme fusion was administered to rats during a 7-day nicotine infusion, it reduced signs of withdrawal following termination of the nicotine infusion compared to the control group. A significant impact was observed on nicotine’s behavioral effects by preventing the development of irritability-like behavior, hyperalgesia and somatic signs of withdrawal in animals exposed to chronic nicotine, strongly supporting the theory that NicA2 may prevent the development of addiction-like behavior. Moreover, there was no nicotine detected in the blood or brains in the treated group, while the control group exhibited expected concentrations of nicotine in both blood and brains[22]. By contrast, nicotine vaccines were only partly able to reduce brain nicotine concentrations, probably leading to the observed lack of efficacy [23].
Importantly, a NicA2-ABD fusion was shown to decrease nicotine discrimination and reductions in nicotine reinforcement in a continuous nicotine access self-administration model which closely resembles human nicotine exposure. In this model [24], rats are trained to self-administer nicotine and develop a stable dependence on nicotine for several weeks before being tested. After a high dose of NicA2-ABD (70 mg/kg), nicotine-seeking behaviour was extinguished over 6 days of testing with rats having continuous access to nicotine as seen in figure 2 [17]. Furthermore, NicA2-ABD decreased compulsive-like nicotine intake, and prevented nicotine- and stress–induced relapse [25].While these initial studies provide proof of concept for the use of NicA2 as a basis for developing a smoking cessation drug, one must obviously be careful with extrapolating these animal-based observations. Limitations of these studies include nicotine dosing by bolus intravenous injection as opposed to inhalation.
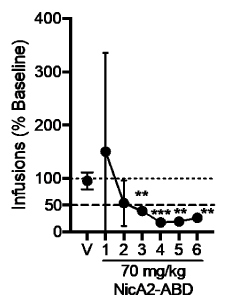
Figure 2. Effects of NicA2-ABD on self-administration of nicotine in rats having unlimited access to nicotine. Mean (± SD) number of infusions during 23-hr access following pre-treatment with PBS vehicle (V) and NicA2-ABD over six consecutive test sessions, expressed as a percentage of baseline. Each point represents the mean of four rats. The dotted horizontal line represents baseline. The dashed horizontal line represents the 50% reduction criterion for extinction. Different from V by paired t-tests, **p < 0.01, ***p < 0.001. Reproduced with permission from [18].
Important challenges will have to be addressed in order to develop NicA2 into an effective therapeutic agent. It needs to be confirmed that the metabolites of NicA2’s nicotine degradation do not lead to significant toxicities. The safety of NicA2 has been initially assessed in mice by administering pseudooxynicotine (PON), the main metabolite of nicotine’s metabolism by NicA2. PON has not been reported to possess addictive properties and is metabolized to a non-toxic keto acid, while all mice remained healthy and post-mortem studies showed no organ damage or neoplasia [14]. Importantly, NicA2 production of PON does not introduce any novel compounds, as 7–9% of the nicotine in smokers is metabolized to PON by liver cytochrome P450 enzymes [25-27]. Screening an initial panel of endogenous nicotine metabolites indicated that NicA2 is highly specific for nicotine [17]. However, more thorough toxicity and safety studies are needed to evaluate the metabolites of NicA2 further, as well as potential side effects related to substrate specificity and potential effects on endogenous substrates.
NicA2 which is a bacterial protein may be immunogenic, since this is unfavorable for human use, this characteristic should be minimized. It should be noted that many engineered proteins of entirely non-human origin or animal-human chimera have been approved by regulatory authorities for human use [28]. In addition, the expected duration of enzyme treatments needed for smoking cessation is relatively brief, as is commonly observed with the 12-week treatment period of present smoking cessation drugs. This relatively short expected duration of treatment should reduce the risk of developing anti-NicA2 antibodies.
Finally, improving catalytic activity to reduce the dose amounts predicted on the basis of the reported in vivo studies will be important. Modern protein engineering techniques have been successful in optimising catalytic activities of enzymes [29,30] . Such improvements are needed for realistic dose levels, suitable routes and frequency of drug administration.
NicA2 promises a new treatment strategy in smoking cessation, since it rapidly degrades nicotine within a few minutes. In a clinical sense, it is envisioned to be administered intravenously by injection in a physician’s office during cessation counselling. As one of the most important predictors of success is self-motivation, it could be an important advantage over vaccines if patients can immediately initiate a quit attempt while the motivational level is at its peak without having to wait for months before an immune response builds up, as in the case of vaccines. Attempts to quit should become easier, since the nicotine does not reach the brain to evoke its rewarding effects and smoking will not result in a rewarding stimulus. Eventually, this process could lead to long-term abstinence, as smoking is no longer associated with a reward.
It is interesting to note the parallel between NicA2 treatment to reduce nicotine exposure and the effect of lowering the nicotine content in cigarettes themselves. In clinical studies, smokers who did not have an intention to quit were provided with a range of nicotine-containing cigarettes, including Very Low Nicotine Content (VLNC) cigarettes (0.3 mg/cig; 2% of Normal Nicotine Content (NNC) cigarettes). These smokers experienced reduced nicotine exposure and dependence, reduced cravings during abstinence from smoking and increased unprompted quit attempts in comparison with smokers who were assigned NNC cigarettes [31]. In treatment-seeking smokers, greater reductions in nicotine exposure while smoking VLNC cigarettes predicted smoking cessation [32]. Furthermore, withdrawal symptoms were mild to moderate, comparable to withdrawal symptoms when using a nicotine patch [33]. Moreover, minimal compensatory smoking was observed [34]. In a study where subjects had free access to VLNC cigarettes but not NNC cigarettes (sequestered in a hotel), a 92–94% reduction in nicotine exposure biomarkers was observed [35]. This approach has recently been embraced in the US by the FDA’s Center for Tobacco Products, proposing the regulation of tobacco products with the intention of lowering the nicotine content to non-addictive levels [36]. Whether it will ultimately be politically possible to eliminate the sale of tobacco containing addictive levels of nicotine is unknown. The timeline for the FDA plans is not yet defined and may take a decade or more to implement fully in the US, while it is uncertain how many other countries worldwide would or could follow a similar path. However, these clinical studies do emphasise that nicotine is a key addictive component and lend validity to the concept of enzymatically eliminating nicotine in the form of smoke inhalation to combat tobacco addiction.
Enzymatic degradation of nicotine appears to be more potent than nicotine-specific antibodies or vaccines for reducing nicotine distribution to the brain in rats. If this situation proves to be the case in humans as well, it could be more effective in order to enhance smoking cessation rates and succeed where nicotine vaccines have failed thus far. This development could be a major step forward in the race that we must win to reduce the number of smokers which die prematurely each year [37,38].
Onno van Schayck has received consulting compensation for advice with respect to smoking cessation clinical study design and development planning as part of the NIH/NIDA Strategic Alliance grant to develop nicotine-specific monoclonal antibodies for smoking cessation (R01DA038877; Kalnik/PI, Antidote Therapeutics, Inc.).
Thomas Thisted and Matthew Kalnik are employees and shareholders of Antidote Therapeutics. TT and MK are inventors on nicotine-degrading enzyme patent applications assigned to Antidote Therapeutics. [16] Bo van Engelen interned at Antidote Therapeutics.
- GBD 2015 Tobacco Collaborators (2017) Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 389: 1885-1906. [Crossref]
- Cahill K, Stevens S, Perera R, Lancaster T (2013) Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev: CD009329. [Crossref]
- Quaak M, van Schayck OCP, Knaapen AM, van Schooten FJ (2009) Implications of gene–drug interactions in smoking cessation for improving the prevention of chronic degenerative diseases. Mutation Research 667: 44-57.
- Roberts E, Eden Evins A, McNeill A, Robson D (2016) Efficacy and tolerability of pharmacotherapy for smoking cessation in adults with serious mental illness: a systematic review and network meta-analysis. Addiction 111: 599-612. [Crossref]
- Molyneux A (2004) Nicotine replacement therapy. BMJ 328: 454-456. [Crossref]
- Hays JT, Ebbert JO, Sood A (2008) Efficacy and safety of varenicline for smoking cessation. Am J Med 121: S32-42. [Crossref]
- Roddy E (2004) ABC of smoking cessation: bupropion and other non-nicotine pharmacotherapies. BMJ 328: 509 [Crossref]
- Fiore M C, Jaen C R, Baker T, Bailey W C, Benowitz N L, Curry, (2008) Treating tobacco use and dependence: 2008 update. Rockville, MD: US Department of Health and Human Services. [Crossref]
- Bellou V, Belbasis L, Konstantinidis A, Evangelou, E (2017) Tobacco smoking and risk for idiopathic pulmonary fibrosis: a prospective cohort study in UK Biobank. European Respiratory Society 50.
- Hatsukami D, Jorenby D, Gonzales D, Rigotti N, Glover E, et al. (2011) Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther 89: 392-399. [Crossref]
- Cornuz J, Zwahlen S, Jungi W, Osterwalder J, Klingler K, et al. (2008) A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One 3: 2547.
- Kalnik M (2016) Therapeutic Vaccines for Treating Nicotine Addiction. In: Montoya ID, editor. Biologics to Treat Substance Use Disorders: Vaccines, Monoclonal Antibodies, and Enzymes. 1st ed.: Springer.
- van Schayck OC, Horstman K, Vuurman E, de Wert G, Kotz D (2014) Nicotine vaccination--does it have a future? Addiction 109: 1223-1225. [Crossref]
- Xue S, Schlosburg JE, Janda KD (2015) A new strategy for smoking cessation: characterization of a bacterial enzyme for the degradation of nicotine. J Am Chem Soc 137:10136-10139. [Crossref]
- Yu H, Tang H, Wang L, Yao Y, Wu G, et al. (2011) Complete genome sequence of the nicotine-degrading Pseudomonas putida strain S16. J Bacteriol 193: 5541-552. [Crossref]
- Janda KD, Kalnik MW, Thisted T, inventor; The Scripps Research Institute and Antidote Therapeutics, Inc., assignee. Nicotine-degrading enzymes for treating nicotine addiction and nicotine poisoning patent PCT/US2016/045109 WO/2017/023904.
- Pentel PR, Raleigh MD, LeSage MG, Thisted T, Horrigan S, et al. (2018) The Nicotine-degrading enzyme NicA2 reduces nicotine levels in blood, nicotine distribution to brain, and nicotine discrimination and reinforcement in rats. BMC Biotechnol 18: 46 [Crossref]
- Rose JE, Mukhin AG, Lokitz SJ, Turkington TG, Herskovic J, et al. (2010) Kinetics of brain nicotine accumulation in dependent and nondependent smokers assessed with PET and cigarettes containing 11C-nicotine. Proc Natl Acad Sci U S A 107: 5190-5195. [Crossref]
- Pentel PR, Malin DH, Ennifar S, Hieda Y, Keyler DE, et al. (2000) A Nicotine Conjugate Vaccine Reduces Nicotine Distribution to Brain and Attenuates Its Behavioral and Cardiovascular Effects in Rats. Pharmacology Biochemistry and Behavior. 65: 191-198.
- Keyler DE, St Peter J, Hieda Y, Pentel PR (1999) Altered disposition of repeated nicotine doses in rats immunized against nicotine. Nicotine Tob Res 1: 241-249. [Crossref]
- Jonsson A, Dogan J, Herne N, Abrahmsen L, Nygren PA (2008) Engineering of a femtomolar affinity binding protein to human serum albumin. Protein Eng Des Sel 21: 515-527. [Crossref]
- Xue S, Kallupi M, Zhou B, Smith LC, Miranda PO, et al. (2018) An enzymatic advance in nicotine cessation therapy. Chem Comm 54:1686-1689.
- Goniewicz ML, Delijewski M (2013) Nicotine vaccines to treat tobacco dependence. Hum Vaccin Immunother 9: 13-25. [Crossref]
- LeSage MG, Burroughs D, Muelken P, Harris AC (2016) Self-administration of smokeless tobacco products in rats. Tob Regul Sci. 2: 329-342. [Crossref]
- Benowitz NL, Hukkanen J, Jacob P 3rd. (2009) Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol (192): 29-60. [Crossref]
- Hecht SS, Hochalter JB, Villalta PW, Murphy SE (2000) 2'-Hydroxylation of nicotine by cytochrome P450 2A6 and human liver microsomes: formation of a lung carcinogen precursor. Proc Natl Acad Sci U S A 97: 12493-12497. [Crossref]
- Von Weymarn LB, Retzlaff C, Murphy SE (2012) CYP2A6- and CYP2A13-Catalyzed Metabolism of the Nicotine ?5'(1') Iminium Ion. J Pharmacol Exp Ther 343: 307-315 [Crossref]
- Lutz SB, Bornscheuer UT (2012) Protein Engineering Handbook. Weinheim, Germany: Wiley-VCH
- Bar-Even A, Noor E, Savir Y, Liebermeister W, Davidi D, et al. (2011) The Moderately Efficient Enzyme: Evolutionary and Physicochemical Trends Shaping Enzyme Parameters. Biochemistry 50: 4402-4410. [Crossref]
- Cobb RE, Sun N, Zhao H (2013) Directed evolution as a powerful synthetic biology tool. Methods 60: 81-90. [Crossref]
- Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, et al. (2015) Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med 373: 1340-1349. [Crossref]
- Dermody SS, Donny EC, Hertsgaard LA, Hatsukami DK (2015) Greater reductions in nicotine exposure while smoking very low nicotine content cigarettes predict smoking cessation. Tob Control 24: 536-539. [Crossref]
- Hatsukami DK, Hertsgaard LA, Vogel RI, Jensen JA, Murphy SE, et al. (2013) Reduced Nicotine Content Cigarettes and Nicotine Patch. Cancer Epidemiology, Biomarkers & Prevention 22: 1015-1024.
- Hatsukami DK, Donny EC, Koopmeiners JS, Benowitz NL (2015) Compensatory Smoking from Gradual and Immediate Reduction in Cigarette Nicotine Content. Cancer Epidemiology Biomarkers & Prevention. 24: 472-476. [Crossref]
- Denlinger RL, Smith TT, Murphy SE, Koopmeiners JS, Benowitz NL, et al. (2016) Nicotine and Anatabine Exposure from Very Low Nicotine Content Cigarettes. Tob Regul Sci 2: 186-203. [Crossref]
- Benowitz NL, Henningfield JE (2018) Nicotine Reduction Strategy: State of the science and challenges to tobacco control policy and FDA tobacco product regulation. Prev Med 117: 5-7. [Crossref]
- Van Schayck OCP, Williams S, Barchilon V, Baxter N, Jawad M, et al. (2017) Treating tobacco dependence: guidance for primary care on life-saving interventions. Position statement of the IPCRG. NPJ Prim Care Respir Med 27: 38. [Crossref]


