Purpose of the study: Synthesis of diketomethyl cellulose.
Materials and method: Diketomethyl cellulose was quantitatively prepared by the oxidation of methyl cellulose by potassium permanganate in alkaline medium at pH`s > 12.
Results: The dike to-derivative was characterized by formation of 2,4-dinitrophenyl hydrazone and dioxime derivatives when reacting with dinitrophenyl haydrazine and hydroxyl amine, respectively, as well as by the FTIR spectral bands observed at 1760-1730 cm-1 that characterize to the carbonyl group of α-diketones.
Conclusion: This oxidation product can be used as a dietary fiber and a functional fiber when added to food. In addition, it found that the product has a high affinity for chelation with most of divalent and polyvalent metal ions forming stable coordination biopolymer complexes of methyl cellulose. The product is characterized by its non-toxicity, low cost and high performance. Diketomethyl cellulose can be used effectively for removal of poisonous heavy metal ions such as Sn2+, Cd2+, Hg2+ and Pb2+, Ca2+ along with other divalent and polyvalent metal ions which are contaminated in wastewater and environment.
methyl cellulose, dietary fiber, chelating agent, permanganate
Cellulose is the major component of cell walls in plant. It is considered as a dietary fiber as well as a functional fiber when added to food [1]. Methyl cellulose (MC) is a cellulose ether derivative. It is a water-soluble due to the presence of hydroxyl moieties at C-2 and C-3 positions which prevent extensive hydrogen bonding. It is a hydrophilic macromolecule unless the temperature exceeds that of the lower critical temperature of solution (LCST) of the approximate range 40-70 oC [2]. Therefore, this natural polymer is expected to have advantageous as a dietary fiber in food industry.
The kinetics and mechanisms of oxidation of polysaccharides such as alginates [3], pectates [4], methyl cellulose [5,6], carboxymethyl cellulose [7], carrageenan [8,9] and chondroitin-4-sulfate [10] by alkaline permanganate have been investigated in more details as reported elsewhere. However, the synthesis of the keto-derivatives for the oxidation of products of the studied polysaccharides was reported elsewhere [11,12], it seems that no mention on the synthesis of the methyl cellulose keto-derivatives.
Moreover, it was reported that the high tendency of alginate polysaccharide to form coordination biopolymers with polyvalent metal cations was attributed to the presence of both carboxylate and hydroxyl groups within the monomers [13]. This means that the presence of such groups in particularly the carboxylate groups within the monomers is essential for formation of such coordination biopolymer complexes. Despite the absence of carboxylate groups within the skeleton of the synthesized diketo-methyl cellulose, preliminary experiments indicated its high tendency to form stable coordination biopolymer complexes with di- and polyvalent metal ions.
The above aspects in addition to our interest in the kinetic studies of oxidation of macromolecules by this oxidant [3-10], we have prompted to undertake the present investigation with the aims to synthesize a novel coordination biopolymer precursor as a chelating agent with high affinity for chelating di- and polyvalent metal ions.
Materials
All materials used were of analytical grade. Doubly distilled water was used in all preparations.
Methyl cellulose (MP Biomedicals, LLC) (MC) was used without further purification. The viscosity of 2% solution was found to be 4000 centipoise at 20℃. The molecular weight is about 50.000 Dalton. The preparation of the stock solution of MC was the same as described elsewhere [14]. This procedure was performed by the addition of methyl cellulose powder regent to bidistilled water whilst rapidly stirring the solution to avoid the formation of lumps which swell with difficult. A stock solution of permanganate was prepared, stored and standardized as described elsewhere [15,16]. All other reagents were prepared by dissolving the requisite amounts of the sample in doubly distilled water.
Preparation of diketomethy cellulose (DKMC)
Methyl cellulose powder (5 g) was dissolved in 350 cm3 of deionized water whose pH was previously adjusted to pH ≥ 12 using sodium hydroxide. This process was performed by stepwise addition of the powder MC to the solution while stirring rapidly and continuously to avoid the formation of aggregates. A 150 cm3 solution containing 3.87 g of potassium permanganate and 4.07 g of sodium fluoride was then added stepwise over 2 h to the MC solution. The reaction mixture was stirred for 48 h at room temperature, the formed MnF4 was filtered off, and the solution was concentrated to one-fifth of the original solution using a rotary evaporator. A portion of this concentrated solution was acidified using dilute acetic acid to a pH of ca. 5-6. The resultant solution dried under vacuum, and then subjected to elemental analysis and IR spectroscopy.
The diketo-derivative was characterized by formation of 2,4-dinitrophenyl hydrazone and dioxime derivatives when reacting with dinitrophenyl haydrazine and hydroxyl amine, respectively, as well as by the FTIR spectral bands observed at 1760-1730 cm-1 that characterize to the carbonyl group of a-diketones.
IR-spectra
The IR-spectra were scanned on a Pyc Unicum Sp 3100 spectrophotometer using the KBr disc technique (4000-200 cm-1). ANAL: Diketomethyl cellulose (DKMC) C7H8O5 (172): Calcd (found): C, 48.84 (48.58); H, 4.65 (4.36). IR: 3473 (OH group); 1652-1634 (broad) (C=O of -diketone); 1634 (γas OCO); 1417 (γs OCO) and 1258 cm-1 (C—O—C of MC) [17].
2, 4-Dinitrophenyl hydrazone derivative
ANAL: C19H16O11N8 (532): Cald (found): C, 42.86 (42.66); H, 3.01 (3.0); N, 21.05 (21.1). IR: 3390 (OH group); 3310 (NH of hydrazone); 1680 (C=N of hydrazone); 1200 (C—O—C of MC).
Dioxime derivative
ANAL: C7H10O5N (188): Cald (found): C, 44.68 (44.69); H, 5.32 (5.33); N, 7.45 (7.42). IR: 3300 (OH of COOH and oxime); 1670 (C=N); 1595 (C=O of COOH); 1225 (C—O—C of MC).
The diketo-derivative was also identified by elemental analysis and IR spectral data as described elsewhere [18,19].
Stoichiometry
Under our experimental conditions in the presence of initial [MnO4-] / [MC] in ≥ 2.0 molar ratio, the stoichiometry of oxidation conforms the following stoichiometric equation,
2(C7H12O5)n+ 4MnO4- =2(C7H8O5) n+4MnO2 +4OH-+2H2O + O2 (1)
where C7H12O5 and C7H8NO5 represent the MC and its corresponding diketo-derivative, respectively. The oxidation product was found to be the same in either the presence or absence of nitrogen atmosphere indicating that the oxidation of the formed aldehyde occurs by permanganate ion oxidant rather than by the dissolved oxygen. The oxidation product was identified by the spectral data and elemental analysis [18]. The diketo-derivative was characterized by the formation of 2,4-dinitrophenyl hydrazone and dioxime derivatives as well as by the FTIR spectral bands observed at 1760-1730 cm-1 that characterize to the carbonyl group of α-diketones [17] as shown in Figure 1.
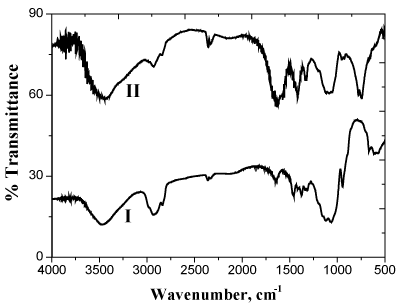
Figure 1. FTIR spectra of methyl cellulose (I) and its dike to derivative (II)
On the other hand, when the molar ration between [MnO4-] /[MC] is 1.0, monoketo-methyl cellulose is formed. Hence, the stoichiometry can be expressed by the following equation:
2(C7H12O5)n+ 2MnO4- =2(C7H10O5) n+2MnO2 +2OH- +H2O+ ½ O2 (2)
Kinetics and mechanism
The kinetics and mechanism for oxidation of MC substrate by alkaline [5,6] and acidic [20] permanganate have been discussed in more details earlier. In case of oxidation of MC by alkaline permanganate [5,6], it was found that the oxidation process was proceeding through two distinct separate stages. The naked-eye observations indicated the change in color of the solution mixture from purple (Mn (VII)) to blue (Mn(V)) to green (Mn (VI)) to yellow (Mn (IV)). The first stage was relatively fast with formation of 1:1 intermediate transient coordination biopolymer complexes involving blue hypomamanganate (V) and/or green manganate (VI) transient species. This stage was followed by slow decomposition of these intermediates to give rise to the final oxidation products as monoketo- or diketo -methyl cellulose precursor derivatives in the final slow stage depending on the initial concentration molar ratio of reactants. Inner-sphere type of the electron transfer mechanism without free-radical intervention was suggested. On the other hand in case of oxidation of MC by acidic permanganate, the oxidation process was found also to proceed via two distinct stage of sigmoidal S-shape nature for pseudo first-order plots. The first stage was found to be relatively slow via formation of 1:2 intermediate complexes prior to the rate-determining step. It corresponds to the formation of substrate radical and Mn3+ and/or Mn4+ transient species as initial oxidation products. This stage was followed by a fast reaction to give rise to keto-derivatives of methyl cellulose in the second fast stage. The mechanisms of oxidation of methyl cellulose by acidic [5,6] and alkaline [20] permanganate is illustrated in Figures 2 and 3, respectively.
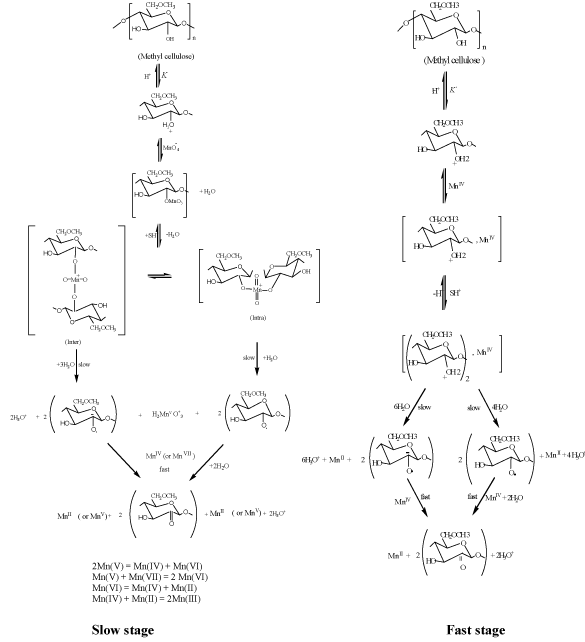
Figure 2. Mechanism of oxidation of methyl cellulose by permanganate ion in aqueous perchlorate solutions for the slow and fast stages
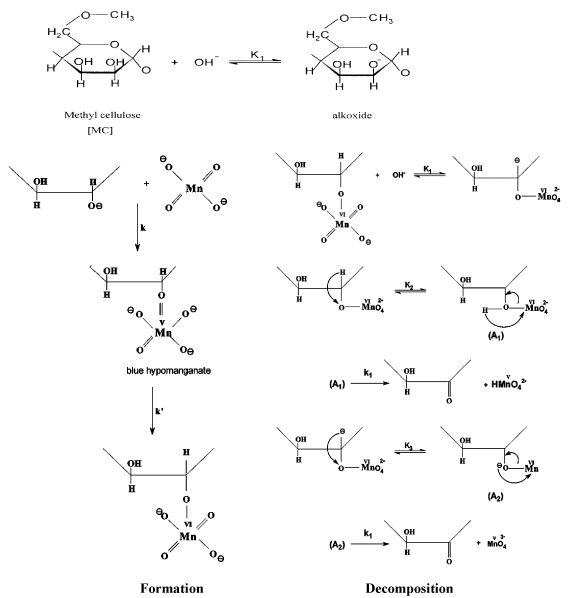
Figure 3. Mechanism of oxidation of methyl cellulose by permanganate ion in alkaline solutions
Chelation geometry with metal ions
It was found that the oxidation product under our experimental conditions (diketo-methyl cellulose derivative) possesses a high tendency to chelate with many metal cations such as silver (I), di-, tri- and tetravalent metal ions, whereas the MC itself does not chelate with these metal ions. The characteristics and geometrical configuration of these complexes are in progress in our laboratory.
Diketomethyl cellulose can be used as a dietary fiber and a functional fiber when added to food. In addition, it found that the product has a high affinity for chelation with most of divalent and polyvalent metal ions forming stable coordination biopolymer complexes of methyl cellulose. The product is characterized by its non-toxicity, low cost and high performance. Diketomethyl cellulose can be used effectively for removal of poisonous heavy metal ions such as Sn2+, Cd2+, Hg2+ and Pb2+, Ca2+ along with other divalent and polyvalent metal ions which are contaminated in wastewater and environment.
No.
- FAO (2017) Aquaculture, the sustainable development goals (SDGs)/Agenda 2030 and FAO’s common vision for sustainable food and agriculture. COFI sub-committee on aquaculture. Ninth session, COFI: AQ /IX/2017/5, Rome, pp: 24-27.
- Karplus I, Hulata G, Ovadia D, Jaffe R (1992) Social control of growth in Macrobrachium rosenbergii. III. The role of claws in bull run interactions. Aquaculture 105: 281-296.
- Mitra G, Mukhopodhay PK, Chattopadhyay DN (2005) Nutrition and feeding in freshwater prawn (Macrobrachium rosenbergii) farming. Aqua feeds: Formulation and Beyond 2: 17-19.
- Langer S, Bakhtiyar Y, Lakhnotra R (2011) Replacement of fishmeal with locally available ingredients in diet composition of Macrobrachium dayanum. Afri J Agricult Res 6: 1080-1084.
- Kaushik SJ, Cravedi JP, Lalles JP, Sumpter J (1995) Partial or total replacement of fish meal by soybean protein on growth, protein utilization, potential estrogenic or antigenic effects, cholesterolemia and flesh quality in rainbow trout, Oncorhynchus mykiss. Aquaculture 133: 257-274
- Goda AM, El‐Haroun ER, Chowdhury K (2007) Effect of totally or partially replacing fish meal by alternative protein sources on growth of African catfish Clarias gariepinus (Burchell, 1822) reared in concrete tanks. Aquacult Res 38: 279-287.
- Hernandez MD, Martínez FJ, Jover M, Garcia Garcia B (2007) Effects of partial replacement of fish meal by soybean meal in sharpsnout seabream (Diplodus puntazzo) diet. Aquaculture 263: 159-167.
- Muin H, Taufek NM, Abiodum RA, Yusof HM, Razak SA (2015) Effect of partial and complete replacement of fishmeal with mushroom stalk meal and soy bean on growth performance of nile tilapia, Orechromis niloticus fingerlings. Sains Malaysiana 44: 511-516.
- Deborah Paripuranam T, Divya VV, Ulaganathan P, Balamurugan V. Umamaheswari S, et al. (2011) Replacing fish meal with earthworm and mushroom meals in Practical diets of practical diets of labeo rohita and hemigrammus Caudovittatus fingerlings. Indian J Anim Res 45: 115-119.
- Zhang D, Zhang Y, Liu BO, Jiang YI, Zhou Q, et al. (2017) Effect of replacing fish meal with fermented mushroom bran hydrolysate on the growth, digestive enzyme activity and an‐ tioxidant capacity of allogynogenetic crucian carp (Carassius auratus gibelio). Turk J Fish Aquat Sci.
- Finkel T, Holbrook NK (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408: 239-247.
- Mattila P, Konko K, Eurola M, Pihlava JM, Astola J, et al. (2001) Contents of vitamins, mineral elements, and somephenolic compounds in cultivated mushrooms. J Agricul Food Chemist 49: 2343-2348.
- Heleno SA, Barros L, Sousa MJ, Martin A, Ferreira ICFR (2009) Study and characterization of selected nutrients in wild mushrooms from Portugal by gas chromatography and high performance liquid chromatography. Microchem J 93: 195-199.
- Carbonero ER, Ruthes AC, Freitas CS, Utrilla P, Galvez J, et al. (2012) Chemical and biological properties of a highly branched β- glucan from edible mushroom Pleurotussajor-caju. Carbohydrate Polymers J 90: 814-819.
- Sajon SR, Sana S, Rana S, Mushiur Rahman SM, Mostarin Nishi Z (2018) Mushrooms: Natural factory of anti-oxidant, anti in flammatory, analgesic and nutrition. J Pharmaco Phytochem 7: 464-475.
- Dalmo RA, Seljelid R (1995) The immune modulatory effect of LPS. Laminran and sulphated laminaran [β(1,3)-D-glucan] on the Atlantic salmon, Salmo solar [L.], macrophages in vitrology. J Fish Diseases 18: 175-185.
- Raa J (1996) The use of immune stimulatory substances of fish and shellfish farming. Rev Fish Sci 4: 229-288.
- Zhang M, Cui SW, Cheung PCK, Wang Q (2007) Antitumor polysaccharides from mushroom: a review on their isolation, process, structural characteristics and anti tumour activity. Trends Food Sci Technol 18: 4-19.
- Ullah MI, AkhtarM, Awais MM, Anwar MI, Khaliq K (2018) Evaluation of immunostimulatory and immunotherapeutic effects of tropical mushroom (Lentinus edodes) against eimeriasis in chicken. Tropical Animal Health and Production 50: 97-104.
- Shrivastava M (1998) Studies on Mushroom Dehydration (Pleurotus florida), Ph.D. Thesis submitted to IIT, KGP, W.B., India.
- Mehta BK, Jain SK, Sharma GP, Doshi A, Jain HK (2011) Cultivation of button mushroom and its processing: an Techno-economic feasibility. Int J Adv Biotechnol Res 2: 201-207.
- Shiuan C, Sheryl P, Gene H, Sharon K, Jingjing Y, et al. (2005) Breast cancer prevention with phytochemicals in mushrooms. Proc Amer Assoc Cancer Res 46: 5186.
- Arianne V, Julian Renato GR, Fumio E (2018) Agro-Industrial waste conversion into medicinal mushroom cultivation. Ref Module Earth Systems Environmen Sci.
- Winer EP, Hudis C, Burstein HJ (2002) American society of clinical oncology technology assessment of the use of aromatase inhibitors as adjuvant therapy for women with hormone receptor positive breast cancer: Status report. J Clin Oncol 20: 3317-3127.
- Dhamodharan G, Mirunalini S (2010). A novel medicinal characterization of Agaricus bisporus (white button mushroom). Pharmacol Online 2: 456-463.
- Volman JJ, Mensink RP, van Griensven LJ, Plat J (2010) Effects of aglucans from Agaricus bisporus on ex vivo cytokine production by LPS and PHA-stimulated PBMCs; a placebo-controlled study in slightly hypercholesterolemic subjects. European J Clinical Nutri 64: 720-726.
- Ozturk M, Duru ME, Kivrak S, Dogan NM, Turkoglu A, et al. (2011) In vitro Antioxidant, anticholinesterase and antimicrobial activity studies on three Agaricus species with fatty acid compositions and iron contents: A comparative study on the three most edible mushrooms. Food Chem Toxicol 49: 1353–1360.
- Mao Y, Mao J, Meng X (2013) Extraction optimization and bioactivity of exopolysaccharides from Agaricus bisporus. Carbohydrate Polymers 92(2): 1602-1607.
- Ghahremani-Majd H, Dashti F (2015) Chemical composition and antioxidant properties of cultivated button mushrooms (Agaricus bisporus). Horticulture Environ Biotechnol 56: 376-382.
- Ndungutse V, Mereddy R, Sultanbawa Y (2015) Bioactive prosperities of mushroom (Agaricus bisporus) stipe extracts. J Food Processing Preservation 39: 2225-2233.
- Smiderle FR, Ruthes AC, Van Arkel J, Chanput W, Lacomin M, et al. (2011) Polysaccharides from Agaricus bisporus and Agaricus brasiliens is show similarities in their structures and their immunomodulatory effects on human monocytic THP-1 cells. BMC Complementary and Alternative Medicine 11: 58.
- Shao S, Hernandez M, Kramer JKG, Rinke DL, Tsao R (2010) Ergosterol profiles, fatty acid composition, and antioxidant activities of button mushrooms as affected by tissue Part and developmental stage. J Agricul Food Chem 58(22): 11616-11625.
- Trease GE, Evans WC (1989) Pharmacology 11th Edn. Bailliere Tindall Ltd. London 60-75.
- Vandendool H, Kratz PD (1963) A generalization of the retention index system including liner temperature programmed gas-liquid partition chromatography. J Chromatogr 11: 463-467.
- Castell JD, Tiews K (1980) Report on the EIFAC, IUNS and ICES vorking group cn standardisation of Methodology in fish nutrition research. FAO, EIFAC TECH. 36: 24
- AOAC (1995) AOAC Official methods of analysis (16th ed). Official method 985.29: Total dietary fiber in foods-enzymatic gravimetric method, AOAC international Arlington, VA, USA.
- APHA (2005) Standard methods for the examination of water and wastewater, 20th ed. (American public health association, New York).
- Tekinay AA, Davies SJ (2001) Dietary carbohydrate level influencing feed intake, nutrient utilization and plasma glucose concentration in the rainbow trout, Oncorhynchus mykiss. Turk J Vet Anim Sci 25: 657-666.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275. [Crossref]
- Moore S, Stein WH (1984) Methods in enzymol (Eds: Olowick, Spand Kalpan, ND), Academic press, New York, pp: 468.
- Roe JH (1955) The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem 212: 335-343. [Crossref]
- Folch J, Lees M, Stanely GHB (1957) A Simple method for the isolation and purification of total lipids from animal tissues. J Bios Chem 266: 497-509.
- Barnes H, Blackstock J (1973) Estimation of lipids in marine animals and tissues. Detail investigation of the sulpho-phosphovanillin method for total lipids. J Experiment Mar Biol Ecol 12: 103-118.
- Furne M, Hidalgo MC, Lopez A, Garcia-Gallego M, Morales AE, et al. (2005) Digestive enzyme activities in Adriatic sturgeon Acipenser naccarii and rainbow trout Oncorhynchus mykiss. A comparative study. Aquaculture 250: 391-398.
- Bernfeld P (1955) Amylases, in: Colowick SP, Kaplan NO. (Eds.), Methods in enzymology. Academic Press, New York, pp: 149-158.
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685. [Crossref]
- Hess B, Sherma J (2004) Quantification of arginine in dietary supplement tablets and capsules by silica gel high performance thin-layer chromatography with visible mode densitometry. Acta Chromatogra 14: 60-69.
- Nichols DS, Nichols PD, Mc Meekin TA (1993) Poly unsaturated fatty acids in Antarctic bacteria. Antarct Sci 5: 149-160.
- Debnath M, Nandi M, Biswas M (2014) A critical pharmacognostic evaluation and preliminary phytochemical investigation of Alternanthera sessilis (L.) R. BR. Leaves. Indian J Pharmaceut Sci Res 4: 71-74.
- Sivakumar R, Sunmathi D (2016) Phytochemical screening and antimicrobial activity of ethanolic leaf extract of Alternanthera sessilis (L.) R. BR. EX DC and Alternanthera philoxeroides (MART.) Griseb. Europ J pharmaceut 3: 409-412.
- Bhushan MS, Rao CHV, Ojha SK, Vijayakumar M, Verma A (2010) An analytical review of plants for anti diabetic activity with their phytoconstituents and mechanism of action. Int J Pharmaceut Sci Res 1: 29-46.
- Latha M, Pari L (2003) Anti hyperglycemic effect of Cassia auriculata in experimental diabetes and its effect on key metabolic enzymes in carbohydrate metabolism. Clin Experimen Pharmacol Physiol 30: 38-43.
- Kalaiselvi VC, Saravana Bhavan P, Kalpana R, Rajkumar G, Satgurunathan T (2018) Phyllanthus amarus enriched Artemia nauplii enhanced survival, growth and nutritional quality of early post-larvae of the prawn Macrobrachium rosenbergii. Clin Nutr Metab 1: 1-15
- Prabhadevi V, SathishSahaya S, Johnson M, Venkatramani B, Janakiramana N (2012). Phytochemical studies on Allamanda cathartica L. using GC–MS. Asian Pacific J Tropical Biomedicine 2: S550-S554.
- Markkas N, Govindharajalu M (2015) Determination of phytocomponents in the methanolic extract of Mollugo cerviana by GC-MS analysis. Int J Res Biol Sci 5: 26-29.
- Sivakumar MVK, Motha R, Wilhite D, Qu J (2011) Towards a compendium on national drought policy: Proceedings of an expert meeting. American meteorological society.
- Diana HY, Parthipan BN (2015) Compounds identification from hypersaline Oscillatoria Salina using GC-MS Analysis. Int J Res Stud Biosci 3: 6-10.
- Priya EP, Subhashini S (2016) Thin layer and deep bed drying basic theories and modeling. Agric Eng Int 18: 314-325.
- Hassan MM, Oyewale AO, Amupitan JO, Abduallahi MS, Okonkwo EM (2004) Preliminary phytochemical and antibacterial investigation of crude extracts of the root bark of Detarium microcarpum. J Chem Soci Nigeria 29: 26-29.
- Kumar A, Mazzanti M, Mistrik M, Kosar M, Beznoussenko GV, et al. (2014) ATR mediates a checkpoint at the nuclear envelope in response to mechanical stress. Cell 158: 633-646. [Crossref]
- Chandrasekaran S, Gaurav G, Serino S, Miranda (2011) Ringing and springing response of triangular TLPs. International Shipbuilding Progress 58: 141-163.
- Venkata Raman B, Samuel LA, Pardha Saradhi M, Narashimha Rao B, Vamsi Krishna AN, et al. (2012) Antibacterial, Antioxidant Activity and GC-MS Analysis of Eupatorium odoratum. Asian J Pharmaceut Clini Res 5: 99-106
- Sudha T, Chidambarampillai S, Mohan VR (2013) GC-MS Analysis of bioactive components of aerial parts of Fluggea leucopyrus Willd (Euphorbiaceae). J Appl Pharmaceut Sci 3: 126-130.
- Yu Y, Lee C, Kim J, Hwang S (2005) Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnology Bioengineering 89: 670-679
- Swamy DN (1995) Traning manual, short-term course in Biotechnological approaches in prawns and fish Nutrition and Feed Technology. CIBA, Madras 82-88.
- Gimenez Ana F, Díaz Ana C, DíazSusana C, Susana V, Jorge L, et al. (2009) Partial substitution of fishmeal by meat and bone meal, soybean meal, and squid concentrate in Feeds for the prawn Artemesia longinaris: Effect on digestive proteinase. Isr J Aquac 61: 48-56.
- Mukhopadhyay PK, Rangacharyulu PV, Mitra G, Jana BB (2003) Applied nutrition in freshwater prawn, Macrobrachium rosenbergii culture. J Appl Aquacult 13: 317-340.
- Aarumugam P, Bhavan PS, Muralisankar T, Manickam N, Srinivasan V, et al. (2013) Growth of Macrobrachium rosenbergii fed with mango seed kernel, banana peel and papaya peel incorporated feeds. Int J App Biol Pharm Tech 4: 12-25.
- Fang LS, Tang CK, Lee DL, Chen IM (1992) Free amino acid composition in muscle and hemolymph of the prawn Penaeus monodon in different salinities. Nippon Suisan Gakk 58: 1095-1102.
- Wilson RP (2002) Amino acids and protein. In: Halver JE, Hardy RW (Eds.), Fish Nutrition. Academic press, San Diego. CA, USA, pp: 143-179.
- Emelyanova LV, Koroleva EM, Savina MV (2004) Glucose and free amino acids in the blood of lampreys (Lampetra fluviatilis L.) and frog (Ranatemporaria L.) under prolonged starvation. Comp Biochem Physiol Part A Mol Integr Physiol 138: 527-532.
- Bhavan PS, Radhakrishnan S, SeenivasanC, Shanthi R, Poongodi R, et al. (2010) Proximate composition and profiles of amino acids and fatty acids in the muscle of adult males and females of commercially viable prawn species Macrobrachium rosenbergii collected from natural culture environments. Int J Biol 2: 107-119.
- Rajkumar G, Saravana Bhavan P, Srinivasan V, Udayasuriyan R, Karthik M, et al. (2017) Partial replacement of fishmeal with marine algae Turbinaria ornata and Gracilaria corticata for sustainable culture of the freshwater prawn Macrobrachium rosenbergii. Int J Res Stud Zool 3: 32-44.
- Radhakrishnan S, Bhavan PS, Seenivasan C, Muralisankar T (2015) Effect of dietary replacement of fishmeal with Chlorella vulgaris on growth performance, energy utilization and digestive enzymes in Macrobrachium rosenbergii post larvae. Int J Fish Aqua 7: 62-70.



