Introduction: Clinical behavior, treatment parameters, and prognostic factors are less well defined in older adults with low-grade gliomas (LGG). We conducted a two-institution retrospective review of older patients with LGG to better understand disease characteristics and prognosis in this population.
Methods: Northwestern University (NU) and The University of Washington (UW) clinical research databases were queried for patients > 50 years of age with a diagnosis of WHO grade II glioma between January 1, 2000 and December 2012 (UW). Medical records were reviewed and data relevant to diagnosis, treatment and outcomes were collected. PFS and OS with respect to prognostic factors were calculated. Log-rank test and multivariate proportional hazards models were calculated for multiple tumor characteristics.
Results: Thirty-five patients with a diagnosis of LGG (WHO grade II) were identified; 15 women and 20 men had a median age of 55 (range 50-78). Fourteen had astrocytomas, fourteen had oligodendrogliomas and seven had oligoastrocytomas. Eight patients had contrast enhancement on neuroimaging, 9 of 21 tested had 1p19q co-deletion and 5 of 14 tested had an IDH1 mutation. Five year PFS was 21% with median PFS of 17 months; 20 patients had died (5 year OS=43%, median OS=48 months). On univariate analysis There was a statistically significant improvement in OS for patients with mixed histology (p=0.001), no midline shift at diagnosis (p=0.002) and with IDH1 mutation (p=0.003),
Conclusion: LGG appear more aggressive in older patients. Treatment following surgical resection should be considered; ongoing studies may clarify the most appropriate treatments for this age group.
low grade glioma, IDH, older patients
The World Health Organization (WHO) classifies low-grade gliomas (LGGs) as grade 1 and grade 2 gliomas. LGGs are more common in younger adults with a peak age of 34 years and clinically behave more aggressively than LGG diagnosed in the pediatric population [1]. In children, grade 2 LGG may be curable with gross total resection alone [2]. and is less likely to dedifferentiate into a higher grade tumor than LGG in adults [3]. This natural course contrasts that of LGG in most adult patients where they invariably dedifferentiate into higher-grade tumors and patients die from their disease [4]. There is not a separate treatment paradigm for older adult patients with LGG. The definition of older versus elderly is somewhat vague with > 40 being high risk in LGG and > 65 elderly in high grade gliomas [5]. There have been few studies of LGG in older patients; the overall survival and progression free survival has been shown to be shorter than younger LGG patients, however specific prognostic factors for this population have yet to be identified [6-8].
For adult patients with LGG, risk factors for poor prognosis were published by Pignatti et al. [9] which reported average survivals of adult patients based on risk factors. The recognized high-risk factors included patient age > 40 years, tumor size >6 cm, tumor crossing midline, persistent neurologic symptoms, and astrocytic histology [6,7]. It was found that patients who possessed > 2 high-risk factors had a lower survival. Several of these risk factors were validated in a North Central Cancer Treatment group trial of 203 LGG treated with high- or low-dose radiation therapy where the survival was only 3.5 years for high-risk patients and 12.6 years for low-risk [4]. Tumor-related risk factors for poor outcome include lack of 1p/19q deletion, no IDH-1 mutation and unmethylated MGMT; the G-CIMP phenotype is a positive prognostic marker in low-grade gliomas [4,10-12]. More recently, Capelle et al found independent factors for poor prognosis in LGG included age ≥ 55 years, an impaired functional status, nonfrontal tumor location, and, most of all, a larger tumor diameter [13]. Pallud found that the velocity of radial expansion was independent predictor of outcome in LGG [5]. Despite several indicators of high-risk status for LGG, the optimal treatment strategy for older patients is unclear. We retrospectively reviewed our data to examine the clinical course of older LGG patients (> 50 years).
Patient selection
This study was approved by the Institutional Review Boards of Northwestern University (NU) and University of Washington (UW). Clinical research databases were queried to identify patients with the following criteria: 1) age > 50 at the time of diagnosis 2) pathology diagnosis of WHO grade II glioma and 3) dates seen and follow up between January 1, 2000 and December 31, 2010 (NU) and 2012 (UW). Data from the each institution’s electronic medical record (EMR) was collected and included symptoms at presentation, MRI imaging at diagnosis and post operatively (extent of resection), tumor histology, treatment details, time to tumor progression and overall survival. The extent of resection was categorized as gross total resection (GTR), subtotal resection (STR) or biopsy only. These factors included age, gender, initial clinical presentation, tumor crossing midline, tumor size, presence of contrast enhancement on neuroimaging, extent of resection, tumor histology and grade, IDH1 mutation and 1p19q deletion status (when available), adjuvant therapy including radiotherapy and chemotherapy.
Statistical analysis
Progression-free survival (PFS) was defined as time from initial surgical diagnosis until unequivocal radiographic or clinical progression. Overall survival (OS) was calculated from the date of surgery until date of death. Prognostic factors were analyzed using proportional hazards regression modeling for association with PFS and OS. PFS and OS were estimated using the Kaplan-Meier method, and were compared between patient cohorts of opposing risk factors by using the log-rank test. PFS analysis was censored for patients with no disease progression and patients who were still alive. OS analysis was censored for patients still alive at last follow-up. All tests were 2-tailed and p<0.05 was considered statistically significant.
Clinical characteristics
Thirty-five patients with LGG > 50 years of age were identified. There were 20 men and 15 women with a median age of 56 (range 50-78). Patient characteristics are listed in table 1. The most common clinical presentation was seizures (42%) and headaches (25%). Less common presenting symptoms included ataxia, blurry vision, and dizziness. Tumor diameter was calculated by taking the cube root of the diameters in the axial and sagittal planes (anteroposterior with a second perpendicular measurement and vertical measurement). The resultant median tumor diameter in our population was 37.0 mm (range 16.9-93.1 mm).
Table 1: Summary of Patient Characteristics (N = 35 )
Characteristic |
Number (%) or median (range) |
Total |
35 |
Age, y |
55 (50-78) |
Sex |
|
Men |
20 (57) |
Women |
15 (43) |
Histology |
|
Astrocytoma |
14 (40) |
Oligodendroglioma |
14 (40) |
Mixed oligoastrocytoma |
7 (20) |
First Clinical Presentation |
|
Headaches |
9 (26) |
Seizures |
14 (40) |
Other (ataxia, blurry vision, dizziness) |
12 (34) |
Tumor Size at Diagnosis (in mm) |
37.0 (16.9-93.1) |
Contrast Enhancement |
|
Yes |
8 (23) |
No |
27 (77) |
Midline Shift |
|
Yes |
3 (9) |
No |
32 (91) |
Extent of Resection |
|
Gross total |
5 (14) |
Subtotal |
15 (43) |
Biopsy Only |
15 (43) |
First Course of Treatment |
|
Radiotherapy + chemotherapy |
14 (40) |
Radiotherapy only |
3 (9) |
Chemotherapy only |
3 (9) |
Observation |
15 (43) |
Median Radiation Dose (in Gy) |
5400 (5400 - 6000) |
Treatment after tumor progression |
|
Resection |
11 (31) |
Chemotherapy |
13 (37) |
Radiotherapy |
10 (29) |
Observation |
3 (9) |
1p19q co-deletion |
|
Yes |
9 (26) |
No |
12 (34) |
Unknown |
14 (40) |
IDH1 mutation status |
|
Yes |
5 (14) |
No |
9 (26) |
Unknown |
21 (60) |
Imaging and pathologic findings
Contrast enhancement was present on initial imaging in 8 (23%) patients; midline shift was present in 3 (9%) patients. Degree of resection was GTR in 5, STR in 15 and biopsy only in 15 patients. Tumor location was frontal in 10, parietal in 4, temporal in 11, frontotemporal in 3, parietooccipital in 2, infratentorial in 2 and diencephalic in 3 patients.
Histologic subtype was astrocytoma in 14 (40%), oligodendroglioma in 14 (40%), and oligoastrocytoma in 7 (20%) patients. 1p19q combined co-deletions were present in 9 (26%) patients, absent or uni-deleted in 12 (34%) and unknown in 14 (40%). IDH1 mutation status was present in 5 (14%) of patients, absent in 9 (26%), and unknown in 21 (60%) of patients.
Treatment
Post operatively, 15 (43%) patients were observed with surveillance serial MRIs every 3-12 months, initially with shorter intervals of 3 months. Post-operative combined radiation and temozolomide was given to 14 (40%), radiotherapy alone to 3 (9%), and chemotherapy alone to 3 (9%) patients. The median radiation dose given was 5400 Gy (range = 5400 – 6000 Gy). At first recurrence, re-resection was performed in 12 patients (44%) at which time 9 patients were shown to progress to higher grade histology; 4 patients transformed to an Anaplastic Astrocytoma, 4 to GBM and 1 was given a diagnosis of pleomorphic HGG. Chemotherapy (Temozolomide in all but one patient who was given PCV) was given to 13 (37%), and radiotherapy given to 10 (28%) patients. Tumor surveillance alone was employed in 3 (9%) patients (two each with a STR or Biopsy) at time of recurrence with serial imaging every 3-4 months.
Patient survival
The median OS for the 35 patients was 48 months (range 30 months to 138 months); twenty patients had died at the time of analysis (Figure 1). The 1-year, 3-year, and 5-year OS were 88.3%, 55.1%, and 43.3% respectively. The median OS by histology was 23 months (10 to 33 months) for astrocytoma, 75 months (41 to 152 months) for oligodendroglioma and not reached for mixed oligoastrocytoma as only 2 of the 7 patients in this group had events (Table 2). The median OS based on extent of resection was 33 months (13 to 87 months) for biopsy only and 41 months (21 to 138 months) for STR (Table 2). Median OS for GTR was not able to be calculated as over 50% survived at time of last follow-up. OS for ages 50-60 (n=22) was 86 m (31-152), 61-70 (n=9) was 29 m and >70 (n=4) was 32 m but there was no significant difference (p=0.19)
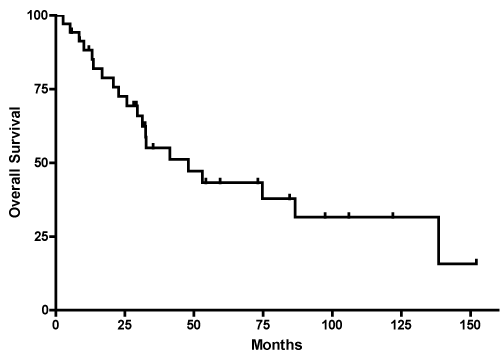
Figure 1: Kaplan-Meier curve shows estimated OS
Table 2: Univariate Analysis of PFS and OS in 35 Patients
|
N |
Overall Survival |
Progression-free Survival |
|
|
Median and range, months |
P |
Median and range, months |
P |
Gender |
|
|
|
|
|
Female |
15 |
33 (17, 152) |
0.70 |
17 (7, 38) |
0.94 |
Male |
20 |
75 (30, 138) |
|
18 (12, 25) |
|
Contrast Enhancement |
|
|
|
|
|
Yes |
9 |
23 (3, 74) |
0.29 |
17 (1, 22) |
0.08 |
No |
26 |
53 (30, 138) |
|
18 (12, 38) |
|
Midline Shift |
|
|
|
|
|
Yes |
3 |
17 (9, 23) |
0.002 |
4 (1, 6) |
0.02 |
No |
32 |
53 (31, 138) |
|
18 (12, 28) |
|
1p19q co-deletion |
|
|
|
|
|
Yes |
9 |
75 (33, 138) |
0.21 |
22 (6, 80) |
0.48 |
No |
11 |
33 (21, 97) |
|
28 (7, 97) |
|
IDH1 mutation |
|
|
|
|
|
Yes |
6 |
N/A |
0.003 |
N/A |
0.06 |
No |
7 |
26 (5, 33) |
|
14 (3, 21) |
|
Extent of Resection |
|
|
|
|
|
Biopsy |
15 |
33 (13, 87) |
0.10 |
14 (7, 22) |
0.004 |
Gross Total Resection |
5 |
N/A |
|
N/A |
|
Subtotal Resection |
15 |
41 (21, 138) |
|
14 (6, 25) |
|
First Course of Treatment |
|
|
|
|
|
Radiotherapy + Chemotherapy |
14 |
41 (14, 138) |
0.10 |
17 (12, 25) |
0.13 |
Radiotherapy Only |
3 |
22 (13, 31) |
|
6.9 (6.7, 7.1) |
|
Chemotherapy Only |
3 |
26 (23, 86) |
|
21 (6, 62) |
|
Observation |
15 |
N/A |
|
18 (8, 121) |
|
*n/a- tissue not available for testing in all patients enrolled
The median PFS for our study population was 18 months, with a range of 12 to 25 months (Figure 2). The 1-year, 3-year and 5-year PFS was 70.6%, 28.9%, and 21.7%, respectively. The median PFS by histological subtype was 12 months (6 to 17 months) for astrocytoma, 25 months (12 to 62 months) for oligodendroglioma, and 38 months (3 to 97 months) for mixed oligoastrocytoma (Table 2). The median PFS for extent of resection was 14 months (7 to 22 months) for biopsy only and 14 months (6 to 25 months) for STR (Table 2); median PFS for GTR was not reached. PFS for ages 50-60 (n=22) was 19 m (8-62), 61-70 (n=9) was 12 m and >70 (n=4) was 18 m but there was no significant difference (p=0.18)
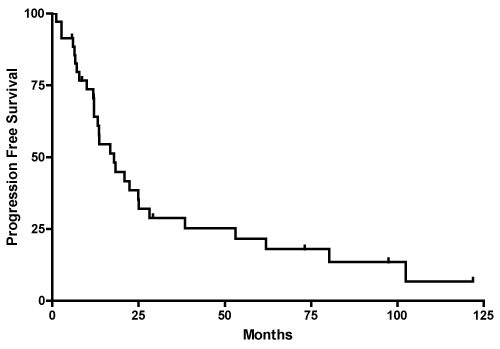
Figure 2: Kaplan-Meier curve shows estimated PFS
2021 Copyright OAT. All rights reserv
Survival with respect to prognostic factors
The results of the univariate prognostic factors are listed in table 2.
The significant prognostic factors for improved OS identified within the cohort were mixed histology subtype (P=0.001), and presence of IDH1 mutation (P=0.003). Those who underwent gross total resection trended towards improved overall survival (p=0.10). Absence of midline shift (P=0.002) also appeared to be associated with improved OS, but it should be noted that there were only three patients in the midline shift group, all of whom died. Median OS was best in patients with frontal lobe tumors (median OS=74 months) compared to patients with parietal (53 mo), temporal (33 mo), diencephalic (12 mo), and infratentorial tumors. This may be related to ability to undergo surgery with a greater extent of resection based on location. There was a longer overall survival in patients who possessed a greater extent of resection and those treated with adjuvant combined radiotherapy and chemotherapy (p=0.10), compared to those receiving either chemotherapy or radiotherapy alone or those who were only observed.
Prognosis measured by PFS was significantly for improved in patients with midline shift on initial neuroimaging (P=0.02), and with greater extent of resection (P=0.004). Absence of contrast enhancement (P=0.08), presence of IDH1 mutation (P=0.06) and post-operative treatment after diagnosis (P=0.13) had a positive trend for improved PFS. The patients who received adjuvant radiotherapy alone had shortened progression free survival than those patients who underwent combined radiotherapy/chemotherapy or chemotherapy alone after diagnosis. Gender and 1p19q co-deletion did not impact PFS in our analysis.
On multivariate analysis, none of the factors analyzed were statistically significant for OS and PFS.
In our retrospective analysis, the median OS was 48 months, which was shorter than that of younger cohorts identified in previous studies.[1,9,14-16] This suggests that LGG in patients > 50 are likely to behave more aggressively and might benefit from upfront treatment. This age-related discrepancy in grade 2 LGG is seen when comparing adult LGG to childhood LGG. Pediatric LGGs overall have a more benign course compared to their adult counterparts and may be cured by surgery alone in some cases [2,3] The influence of age on progression-free survival was also seen in this older cohort. Median PFS was approximately 17 months, which was shorter in other studies of LGG that include all ages of adults [9,17]. Our retrospective analysis confirms findings in prior studies [1,9,18,19], showing a decrease in overall survival and progression-free survival in older LGG patients over the age of 50 compared to patients < 50. One prior long-term analysis from the Mayo Clinic showed an overall survival of older LGG patients (> 55 years) to be 32.4 months; however their cohort had only one patient who underwent a GTR.[1].
Improved outcomes were seen for patients who underwent resection over biopsy, which is in line with prior analyses [16]. Advancements in surgery may allow for a greater extent of resection, which leads to improved survival [20,21]. The presence of midline shift at diagnosis correlated with improved survival although only 3 patients had midline shift so this findings is questionable. The presence of contrast enhancement trended to poor outcome, possibly suggesting lesions in anaplastic transformation or potential sampling error in tumor pathology analysis. The presence of contrast enhancement as a negative predictor for OS has been shown in younger patients with LGG patients.[1,9,18,19].
When available, 1p/19q and IDH mutations were evaluated with respect to overall prognosis. Consistent with prior analysis [5], it was observed in our cohort that the presence of IDH1 mutation status was associated with better OS and PFS (3-year OS for IDH1 (n=7) vs. no IDH1 (n=6): 100% vs. 21%, 3-year PFS for IDH1 (n=7) vs. no IDH1 (n=6): 67% vs. 0%). With respect to 1p19q deletion status, overall survival was improved but was not significantly different [22]. Despite previous studies that indicate that median survival in patients with diagnosed 1p and 19q deletion is twice as long in comparison with patients in whom no deletion was observed, our study did not find such a significant difference [11].
As with all retrospective studies, our analysis of older LGG patients carries selection and information biases that may have impacted our final results. First, we have a small sample size of varying histologies with treatments being performed based on physician preferences. Given the timeline for included patients (2000-2012), many patients did not have routine IDH and MGMT testing done; however, we were able to have IDH and 1p/19q performed on some samples. The limited number of samples for analysis is another limitation and likely accounts for the differences we say in relation to outcomes relative to other published studies. Furthermore, an analysis looking a larger cohort of patients would have been better to assess each analyzed risk factor and their impact on progression-free, as well as overall survival. Finally, while we had some prognostic factors noted on univariate analysis, not remain when analyzed in a multivariate model, which may be due to our small sample size.
The optimal management of older patients with LLG is unclear but the shorter survivals suggest that earlier intervention should be considered. Recently, Shaw et al. completed a randomized trial comparing RT alone to RT plus PCV (procarbazine, CCNU, vincristine) in high risk LGG patients in the RTOG 9802 trial and saw a potential delayed survival advantage with the addition of chemotherapy [15]. A recent press from the National Cancer Institute (NCI) release noted the OS for the RT-PCV arm was 13.3 years compared to 7.8 years for RT alone arm showing that the addition of chemotherapy may benefit OS [17].
This retrospective analysis supports prior studies [1,9,18,19], showing a shorter overall survival and progression-free survival in older LGG patients over the age of 50 compared younger patients with LGG. We identified prognostic factors that may guide treatment within this population; however larger analyses should be conducted to confirm these risk factors. Older patients with LGG have a poorer prognosis and as such they may require more prompt and aggressive treatment. Until a randomized trial is performed in this group of patients treatment with RT and PCV is a rationale approach but using TMZ instead of PCV would also likely be an option but no data exist for it use.
The work is supported by the NIH grants (R01 NS060752, R01 CA164371, U54 CA210180, U54 CA143970, U54 CA193489, U01CA220378) of Dr. Kristin Swanson.
- Schomas DA, Laack NN, Brown PD. (2009) Low-grade gliomas in older patients: long-term follow-up from Mayo Clinic. Cancer 115: 3969-3978. [Crossref]
- Pollack IF, Claassen D, al-Shboul Q, Janosky JE, Deutsch M (1995) Low-grade gliomas of the cerebral hemispheres in children: an analysis of 71 cases. J Neurosurg 82: 536-547. [Crossref]
- Sievert AJ, Fisher MJ (2009) Pediatric low-grade gliomas. J Child Neurol 24: 1397-1408. [Crossref]
- Daniels TB, Brown PD, Felten SJ, Wu W, Buckner JC, et al. (2011) Validation of EORTC prognostic factors for adults with low-grade glioma: a report using intergroup 86-72-51. Int J Radiat Oncol Biol Phys 81: 218-224. [Crossref]
- Blonski M, Pallud J, Gozé C, Mandonnet E, Rigau V, et al. (2013) Neoadjuvant chemotherapy may optimize the extent of resection of World Health Organization grade II gliomas: a case series of 17 patients. J Neurooncol 113: 267-275. [Crossref]
- Pruitt A, Henson JW (2009) Equal care for the elderly with low-grade gliomas? Neurology 73: 2056-2057. [Crossref]
- Pouratian N, Mut M, Jagannathan J, Lopes MB, Shaffrey ME, et al. (2008) Low-grade gliomas in older patients: a retrospective analysis of prognostic factors. J Neurooncol 90: 341-350. [Crossref]
- Kaloshi G, Psimaras D, Mokhtari K, Dehais C, Houillier C, et al. (2009) Supratentorial low-grade gliomas in older patients. Neurology 73: 2093-2098. [Crossref]
- Pignatti F, van den Bent M, Curran D, Debruyne C, Sylvester R, et al. (2002) Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol 20: 2076-2084. [Crossref]
- Figarella-Branger D, Bouvier C, de Paula AM, Mokhtari K, Colin C, et al. (2012) Molecular genetics of adult grade II gliomas: towards a comprehensive tumor classification system. J Neurooncol 110: 205-213. [Crossref]
- Ręcławowicz D, Stempniewicz M, Biernat W, Limon J, Słoniewski P (2013) Loss of genetic material within 1p and 19q chromosomal arms in low grade gliomas of central nervous system. Folia Neuropathol 51: 26-32. [Crossref]
- Houillier C, Wang X, Kaloshi G, Mokhtari K, Guillevin R, et al. (2010) IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology 75: 1560-1566. [Crossref]
- Capelle L, Fontaine D, Mandonnet E, Taillandier L, Golmard JL, et al. (2013) Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. J Neurosurg 118: 1157-68. [Crossref]
- Pouratian N, Schiff D (2010) Management of low-grade glioma. Curr Neurol Neurosci Rep 10: 224-231. [Crossref]
- Ruiz J, Lesser GJ (2009) Low-grade gliomas. Curr Treat Options Oncol 10: 231-242. [Crossref]
- Schomas DA, Laack NN, Rao RD, Meyer FB, Shaw EG, et al. (2009) Intracranial low-grade gliomas in adults: 30-year experience with long-term follow-up at Mayo Clinic. Neuro Oncol 11: 437-445. [Crossref]
- Shaw EG1, Wang M, Coons SW, Brachman DG, Buckner JC, et al. (2012) Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol 30: 3065-3070. [Crossref]
- Legler JM, Gloeckler Ries LA, Smith MA, Warren JL, Heineman EF, et al. (2000) RESPONSE: re: brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst 92: 77a-8. [Crossref]
- Shaw E, Arusell R, Scheithauer B, O'Fallon J, O'Neill B, et al. (2002) Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol 20: 2267-2276. [Crossref]
- Grossman R, Nossek E, Sitt R, Hayat D, Shahar T, et al. (2013) Outcome of elderly patients undergoing awake-craniotomy for tumor resection. Ann Surg Oncol 20: 1722-1728. [Crossref]
- Turkoglu E, Gurer B, Sanli AM, Dolgun H, Gurses L, et al. (2013) Clinical outcome of surgically treated low-grade gliomas: A retrospective analysis of a single institute. Clin Neurol Neurosurg 115: 2508-2513. [Crossref]
- Leu S, von Felten S, Frank S, Vassella E, Vajtai I, et al. (2013) IDH/MGMT-driven molecular classification of low-grade glioma is a strong predictor for long-term survival. Neuro Oncol 15: 469-479. [Crossref]


