Abstract
To ensure the mental well-being of animals in human care, it is necessary to evaluate their welfare. One way to improve the animals’ wellbeing is to introduce cognitive challenges that requires creative problem solving through divergent thinking, openness, tolerance of ambiguity and intrinsic motivation. Cognitive challenges can be provided by performing research activities with the animals. Here, we determine whether engaging in research provides positive mental stimulation in two grey seals (Halichoerus grypus). The effects of participating in research varied between the two individuals depending on their individual personalities. For one of the seals, being involved in the research was motivating and positive, while the second seal displayed a tendency for low motivation and frustration. This indicates that captive animals may react to cognitive challenges differently, not only between species, but also between animals of different personalities.
Key words
animal welfare, marine mammals, psychophysics, personality, enrichment
Introduction
Animal welfare is described as “the state of the animal’s body and mind, and the extent to which its nature (genetic traits manifest in breed and personality) is satisfied” [1]. It is determined by both its physiological and psychological conditions [2]. The variables contributing to animal welfare may vary between individuals, even within the same species, e.g., due to the animal’s age and sex [2]. The word personality can also be used to describe these consistent behavioral reactions to different environmental variables [2].
Ensuring that the lives of animals in human care are properly being enriched has become a key topic over the last few years [3]. Welfare is notoriously difficult to measure [4] and may lie on a scale from bad to good [5]. To understand an animal's welfare, we need to evaluate a combination of behavioural, physiological and biochemical measurements. There is a broad range of behavioural and physiological measures of poor welfare described above, but there are substantially fewer behaviours, and no physiological measures, that are used as indicators of good welfare [5]. The behaviours used to indicate improved welfare are exploratory behaviour, and an increased range of behaviours [6], together with a reduction of behaviours indicative of poor welfare. [7] defines environmental enrichment as the way to enhance positive experiences in captive animals. The goal of enrichment is to improve the target animal’s physical fitness and/or mental well-being, and in other words, its welfare [8,9] . But, if the enrichment is not adapted to the species or individual in question, it may not have these desired effects [6].
It is now widely accepted that good welfare is more than just the absence of a negative experience, but is more related to the presence of positive experiences, such as pleasure [7], and can thus be provided as a form of enrichment. The goal of enrichment is to enhance the quality of captive animal care by identifying and providing environmental stimuli that is necessary for optimal psychological and physiological “well-being” [3]. Captive animals are primarily held in environments that are smaller and less complex than what they would experience in their natural habitat [10,11] . Therefore, enrichment is necessary to ensure that the animal receives the mental and physical stimulation that it would otherwise have in the wild. This enhanced quality of life could be achieved by physically enriching an already varied environment with new resources or via cognitive means [7]. Creating a situation where there is anticipation of a positive reward, by offering more space to promote play, and by providing opportunities for positive contrast situations for improving coping abilities and information gathering, can also be seen as enrichment [7]. Nevertheless, in all cases it is necessary to confirm that the enrichment is having the desired effect, which is not as easy as some might propose [7].
Cognitive challenges have shown to induce positive changes in the welfare of animals [12-14]. For example, [15] cognitive experiment showed that just by introducing call-feeding stations in the pigs housing structure as environmental enrichment will keep the pigs permanently increasing their locomotor activity, probably due to the distribution of food into several small portions per day. Additionally, [16] proved that cognitive experiments worked as efficient food-based enrichment for captive chimpanzees, when comparing wild and captive chimpanzees. Such challenges could either be presented as a form of enrichment to the animal, which may include actual scientific investigations of the animal’s abilities and behavior. Scientific studies have the potential to increase our understanding of animals, as well as how to improve their mental and physical stimulation [17]. For animals with complex cognitive skills, such as marine mammals and apes, providing floating objects as enrichment barely caters to their high cognitive abilities [14], therefore, with the abundance of animal research being conducted in zoos, marine parks, and research facilities, there is a great potential for research to provide meaningful enrichment to the animal participants.
Several studies have examined the positive effects of cognitive experiments on the welfare of the animal subjects involved [13,16,18] with only a few examining the positive effects of other types of research e.g. [19].
Here we investigate if participation in psychophysical research can be considered enrichment for two captive grey seals (Halichoerus grypus), which have very different personalities and life experiences.
Method
Study subjects
The experimental subjects, coded M1 and M2, were two male grey seals (Halichoerus grypus) housed at the Marine Biological Research Centre at the University of Southern Denmark (SDU) in Kerteminde, Denmark. M1 was born in January 2011 (five years old) and arrived at SDU aged two years. Prior to coming to SDU, M1 had never been exposed to environmental enrichment (pers. Comm., Irene Ayllón González). M2 was born in February 2014 (one year old) and arrived at SDU from Kolmården Wildlife Park, Sweden at two months of age. M2 was weaned one week prior to his arrival at SDU and was still in the process of learning how to eat fish. At SDU, both seals were trained four to five times a day by operant conditioning with positive reinforcement (hand-fed) and participated in cognitive behavioural research [20]. M1 began learning the research task two months upon M2’s arrival at the research centre. M2 began training for the research task one month after his arrival. Both seals had no previous training of any kind, regarding participating in behavioural or cognitive research, and therefore were trained four to five times a week for nine months for M1 and seven months for M2 months prior to the start of data collection.
Housing and husbandry
Subjects were housed in a 170m2 open-air enclosure situated on the edge of a marina, consisting of several pools, level decked wooden haul-out areas, and a metal mesh perimeter. There were three circular housing pools: two pools (A: depth: 2 m, width: 4 m, length: 14 m; B: depth: 2 m, diameter: 4 m) and one holding pool (depth: 1 m, diameter: 1.5 m), all containing natural salt water, on an open-circulation system. Each pool was separated from the others by metal mesh fencing, which allowed for the trainers to gate the subjects between pools without the animals coming into direct contact with one another, by opening and closing gates; this allowed the seals to have visual and acoustic contact with each other but prevented aggression. This separation was maintained throughout the data collection period due to both animals being male, and the large size and weight difference between the two animals (average weight during the study period: M1 = 129 kg; M2 = 67 kg).
Diets for each seal were calculated to ensure that the animals maintained a healthy body mass appropriate for their size and the season (breeding, non-breeding, summer, winter). Research took place from January to June 2015. The daily diet was divided amongst the daily training sessions and used as reinforcement. On days when research was conducted, the diets were divided so that 30-50% of the daily diet was allotted for the research session and the remaining 50-70% was divided amongst the remaining 3-4 training sessions. The diets consisted of a mixture of sprat (Sprattus sprattus; 155 calories/100 grams), capelin (Mallotus villosus; 136 calories/100 grams), and herring (Clupea harengus; 190 calories/100 grams). In addition to daily training, a variety of ‘toys’ were provided ad hoc every day as a form of environmental enrichment. Their enrichment value was not were not formally evaluated.
Experimental procedure
Data was collected as part of a larger study investigating underwater hearing thresholds of the grey seal, thereby contributing to our understanding of the effects of anthropogenic noise on this marine mammal species. For the hearing study, the subjects were trained to participate in a psychophysical study with a GO/NOGO testing paradigm, using positive reinforcement procedures [21-23]. Seals were tested individually in the largest pool (14 m x 4 m x 2 m); prior to the research session the subject was gated by being given a vocal and hand cue to leave the pool they were in and to voluntarily enter the research pool. A minimum of two researchers were present for each research session: The experimenter (K.A.H) and the observer (S.T.O. & A.M.). The experimenter reinforced the seal following each correct response, and the observer recorded the progression of trials, quantity of reinforcement (number of fish received), and the seal’s behaviour during and between trials (i.e. response to a trial, swimming away from the station, or playing with fish). The quantity of reinforcement remained constant throughout a single research session. The experimenter sat on the edge of the pool, above the test set-up, and the observer sat approximately 1 meter away (Figure 1). During each session, it was attempted to maintain a quiet environment and little movement on or around the deck was made. This was done to avoid the stimulus tone from being masked, as well as to minimise any distractions that may cause the animal to lose focus during a research session. The experimenter controlled the start and end of each trial with a custom-made handheld console. In addition to controlling the start of each trial, there were buttons that allowed the experimenter to immediately record whether the animal’s response was correct (GO/NOGO) or incorrect (False Alarm/MISS) directly to the computer. Therefore, in addition to the paper records the observer made, there was also a computerized record for each session. To avoid the experimenter from knowing the order of the trials before each session, which could lead to accidental cueing to the animal as to what the correct behaviour should be, two small LED lights were on the front of the handheld console. One LED would illuminate after a trial was initiated, and the other LED would illuminate to indicate if a signal was presented, or remain unlit to show that no signal was presented.
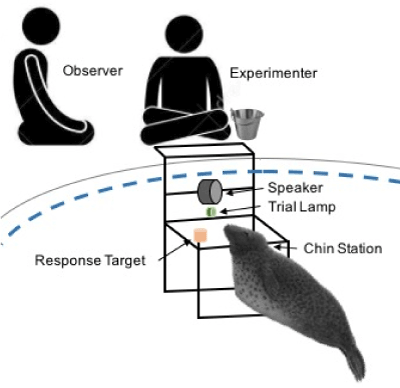
Figure 1. Schematic of underwater setup for psychophysical research.
(Figure 1)
Prior to the start of a trial, the subject was asked to ‘station’ by both a vocal and hand cue: they would then dive to an underwater station, 75 cm below the surface, and place their chin onto the chin-plate. The station was located 50 cm in front of an underwater loudspeaker (UW-30, Electro-Voice, Buchanan Michigan, USA; 20 cm) and a trial signalling light (Figure 1). The trial began when the light turned on, signalling that an auditory tone may/may not follow. The frequency and duration of stimulus was maintained for an entire research session, however, the intensity of the signal changed from one trial to another as the animal continued to respond correctly (See ‘method of limits’: Levitt, 1971; Gescheider, 1997, Chapter 3). Two types of trials were used in this testing procedure: (1) signal present (GO) and (2) signal absent (NOGO). The order in which the trials were presented to the animal were randomized using 12 pre-made trial sequences construced by a Matlab program, using [24] rules for an ‘appropriate’ randomization of psychophysical trials. The Gellerman rules are that the number of stimuli should remain the same to avoid stimulus bias, and that the stimulus is not presented more than three times in a row. The initially completely random schedule was adjusted so that there were never more than three trials in a row of either GO or NOGO trials. For a correct GO trial, the seal would leave the station upon the presentation of an audio signal and touch a red response target to the left of the station with its nose. For a correct NOGO trial, the seal would remain stationed until the trial light was turned off. After each correct response, a bridge was emitted from the underwater speaker, followed by the seal returning to the surface where the trainer would hand feed the fish reward, to ensure that the animal could distinguish between the trial tones and the bridge, the bridge was a 6-kHz broadband tone. The number and type of fish that the seal received varied between the individuals, but the quantity and type of fish remained constant between trials for an entire research session. For incorrect responses, the animal was not bridged and did not receive a fish reward. He was then asked to re-station for the start of the next trial. A ‘time out’ situation was only used if an animal left the station 3-4 times in a row, and lasted 10-15 seconds. The inter-trial interval was attempted to be kept short, but was elongated if an animal left station to swim the pool or played with the fish they had received for reinforcement. Each research session lasted approximately 12-15 minutes. At the start of each session, each animal had a certain number of trials to complete for that session with no time limit to complete that task. Research sessions were conducted once per day, five times per week. These sessions were primarily conducted as the first session of the day, where one animal would be worked at 9 AM and the second animal would then be worked at 9:30 AM.
As stated above, the observer was responsible for recording the seal behaviour during a research session. A seal’s level of motivation towards the research session was determined by the frequency with which the animal left the station and swam away during a research session. Leaving station was defined as when an animal would leave the area around the chin station (>0.5 m) for longer than three seconds. If a seal took more than three seconds to eat the reinforcement, or rejected the reinforcement (e.g. did not take the fish when it was presented to them, or dropped it after receiving it), it was interpreted as low interest in reinforcement. In contrast, high motivation towards the research session was quantified as when the animal both stayed within a radius equal to or less than 0.5 meters from the chin station and returned to it within a time duration equal to or less than three seconds. Interest in the reinforcement was quantified as the animal eating the reinforcement in less than three seconds. In addition to the observer’s notes, the daily calorie intake for each seal was calculated daily. It was then possible to calculate a calorie value per reinforcement, calories consumed during a research session, and calories consumed as reinforcement as a percentage of the total caloric intake for the day. The total number of observations were taken from 32 sessions for M1 and 40 sessions for M2.
Data analysis
Analyses were performed using R Studio software (RCoreTeam, 2015). Seal behaviour was compared between subjects and over time (i.e. research sessions) using a General Linear Model (GLM) fitted with a Gaussian error structure. The response variable was seal behaviour: frequency of leaving the station per session (to indicate low motivation in the task) and playing with or rejecting fish (to indicate low interest in reinforcement). The explanatory variables were the seal’s identity (M1/M2) and the experimental session number (For M1 = 32 sessions and for M2 = 40 sessions).
To test whether the number of trials a subject completed was significantly different across a period of time (experimental sessions), a GLM was fitted with a binomial error structure. The response variable was the number of trials completed per session and the explanatory variables were the seal’s identity and experimental session number.
Ethical Statement
The research followed all applicable international, national, and/or institutional guidelines for the care and use of animals. The animals were kept under Nature Protection Agency Permit SNS-342-00056 and Ministry of Food and Agriculture Permit 2300-50120-00003-09.
Result
Both seals were observed leaving their stations during the research sessions, but the number of times they left per session was significantly higher for M1 than M2 (GLM testing, SD = -3.36, d.f = 70, p = 4e-12; Figure 2). In contrast, the times M2 remained close to the experimental set up was significantly higher than M1 (GLM testing, SD = -3.36, d.f = 70, p = 4e-12; Figure 3). M1 never needed more than three seconds to eat his reinforcement, never rejected it, and always showed a high interest in the reinforcement. M2 showed lower interest in reinforcement during research sessions (GLM testing, SD = 2.32, d.f = 70, p = 0.02; Figure 3). M1, for 100% of the time, ate his reinforcement in less than three seconds, which was significantly different from M2, whose interest in reinforcement was less significant (GLM testing, SD = 2.32, d.f = 70, p = 0.02; Figure 4).
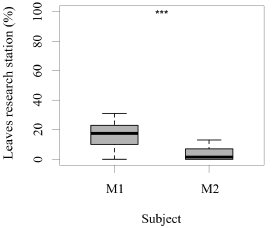
Figure 2. Number of times (%) that each animal left the research station (greater than a 0.5 m radius) after each trial (N = 27 trials/session for M1; N = 31 trials/session for M2; SD= -3.36; *** indicates p < 0.001, ** indicates p < 0.01, * indicates p < 0.05).
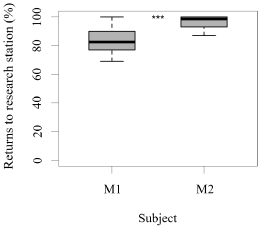
Figure 3. Number of times (%) that each animal remained around the research station (less than a 0.5 m radius) and returned to station in a time span less than three seconds after each trial (N = 27 trials/session for M1; N = 31 trials/session for M2; SD= -3.36; *** indicates p < 0.001, ** indicates p < 0.01, * indicates p < 0.05).
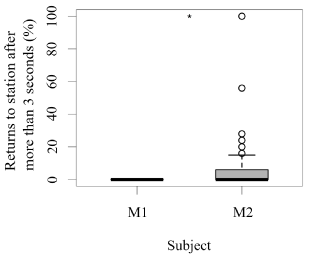
Figure 4. Number of times (%) each animal required more than three seconds to eat their reinforcement after each trial (N = 27 trials/session for M1; N = 31 trials/session for M2; SD= 2.32; *** indicates p < 0.001, ** indicates p < 0.01, * indicates p < 0.05).
There was no significant difference in the number of trials completed per session for either M1 (average 27 trials/session, GLM testing SD= -0.22, d.f= 30, p= 0.6) or M2 (average 31 trials/session; GLM testing SD= 0.13, d.f= 38, p= 0.7). Even though M2 completed 17% more trials/session than M1, this was not significantly different (GLM testing SD= 4.67, d.f= 70, p= 1e-06) (Figure 2).
There was a significant difference between M1 and M2 regarding the total amount of reinforcement received during a research session, which averaged to be 37% and 19% of the individual’s daily caloric intake, respectively (GLM testing SD= -17,94, d.f= 70, p= 2e-11; Figure 3, 4,5,6).
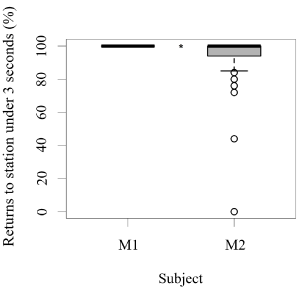
Figure 5. Number of times (%) each animal ate their reinforcement in a time span equal to or less than three seconds after each trial (N = 27 trials/session for M1; N = 31 trials/session for M2; SD= 2.32; *** indicates p < 0.001, ** indicates p < 0.01, * indicates p < 0.05).
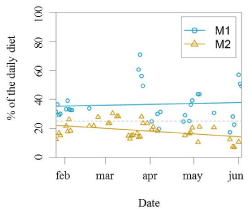
Figure 6. Percentage of the daily diet used as reinforcement per research session for each animal. Coloured lines are derived from the linear model (SD= -17,94). The grey dashed line is situated at the value 25%, as this represents the amount of food they would receive in a regular training
Discussion
Several studies show the positive affect on the welfare of captive primates when cognitive challenges are introduced to them [8,13,14,16,18]; [14], as well as the improved neophobic reactions in captive parrots (Personal communication with Sara Torres Ortiz). It is important to consider that our experimental design can differ from cognitive challenges making the comparison of the two more complicated: during the GO/NOGO testing, if the animal was to respond incorrectly, no reinforcement was given. As the test signal approached the animal’s hearing threshold, the animal may not hear it even when a stimulus was presented and therefore, from their perspective, they are not being reinforced for performing the correct behavior. This lack of reinforcement for what the animal perceived to be the correct behavior can cause frustration. Based on the number of trials the animal participated in for each research session, as well as the amount of reinforcement given per trial, we can assume that M2 was better at dealing with this frustration than M1, if this was considered frustration by M2.
Individual differences, such as personality and life experiences, can affect the animal’s performance when they are presented with a problem they should solve. Several variables have been identified to influence creative problem solving, such as divergent thinking, openness, tolerance of ambiguity and intrinsic motivation [25] . By considering M1’s and M2’s personality and life experiences, we can better interpret their reactions and behavioural trends during research sessions.
The aim of this study was to determine whether engaging in psychophysical research could be used as enrichment and provide positive mental stimulation in two grey seals. Our results indicate that participation in research can affect individuals differently. Psychophysical tasks have been known to provide cognitive challenges for animals [26,27]. Even though both seals studied participated in the research project, our results show that M2 was a well-motivated participant in sensory response research, while M1 was less motivated and required more reinforcement to participate. This may be due to differences in personality and life experiences between the two seals. Such differences between the animals may have a large effect on their motivation to participate in research trials, as well as the enrichment they get out of such participation.
As an individual’s motivation to perform a research task may change the outcome of the study, the variation between M1’s and M2’s view on the significance of reinforcement may be attributed to the animal’s desire to participate in the trials. According to Clark (2011), cognitive enrichment is “a task (or tasks) which (1) engages evolved cognitive skills by providing opportunities to solve problems and control some aspect of their environment, and (2) is correlated to one or more validated measures of wellbeing”. M2 would intermittently refuse reinforcement and return directly to the start position for the next trial. In some instances, he would continue to participate in the session even though he had no interest in the reinforcement. This suggests that this type of task is enriching for M2 [28]. This voluntary participation in research was never observed with M1, who sometimes received half of his total daily diet during these sessions. M1 required a large amount of reinforcement to participate in the sessions and tended to swim away more often between trials.
In addition to personality, examining the environments in which these animals originated from and their life histories provide information that may have influenced the differences between the two seals in their approach to completing the task presented to them. From a very young age, M2 was encouraged and reinforced for being creative and exhibiting play behaviour as well as being introduced to new and different situations. In contrast, during M1’s first three years, he was not actively encouraged to be creative or introduced to new and challenging situations.
The results indicate that it can be helpful to consider the animal’s personality (i.e. creativity, frustration tolerance, intrinsic motivation and curiosity) when evaluating if a given research project can be considered enriching to the individual. Additionally, it is also important to consider the species’ behavioural characteristics. For example, while captive cetaceans are known by their interest in novel objects [29,30], other animals such as ravens [31] or parrots [32] are neophobic and can easily become stressed when introduced to new objects or experimental setups. Such variations in intra- as well as interspecific behaviours may not only have large consequences for animal welfare, but also for the scientific results that the research project produces.
References
- Duncan IJ, Fraser D (1997) Understanding animal welfare.
- Hosey G, Melfi V, Pankhurst S (2009) Zoo Animals-Behaviour. Management, and Welfare. Oxford University Press.
- Shepherdson D (1998) Tracing the path of environmental enrichment in zoos. Second nature: Environmental enrichment for captive animals 1-12.
- Mason G, Mendl M (12021 Copyright OAT. All rights reservasuring animal welfare? Animal welfare 2: 301-319.
- Bassett L, Buchanan-Smith HM (2007) Effects of predictability on the welfare of captive animals. Applied Animal Behaviour Science 102: 223-245.
- Young RJ (2003) Environmental enrichment for captive animals. Blackwell Publishing.
- Boissy A, Manteuffel G, Jensen MB, Moe RO, Spruijt B, et al. (2007) Assessment of positive emotions in animals to improve their welfare. Physiology & Behavior 92: 375-397.
- Hunter SA, Bay MS, Martin ML, Hatfield JS (2002) Behavioral effects of environmental enrichment on harbor seals (Phoca vitulina concolor) and gray seals (Halichoerus grypus). Zoo Biology 21: 375-387.
- Swaisgood RR, Shepherdson DJ (2005) Scientific approaches to enrichment and stereotypies in zoo animals: what's been done and where should we go next? Zoo Biology 24: 499-518.
- Chamove A, Anderson J (1989) Examining environmental enrichment.
- Buchanan-Smith H (1997) Environmental control; an important feature of good captive callitrichid environments. Marmosets and Tamarins in Biological and Biomedical Research. DSSD Imagery, Salisbury, UK, 47-53.
- Moberg GP (2000) Biological response to stress: implications for animal welfare. The biology of animal stress: basic principles and implications for animal welfare, 1-21.
- Clark FE (2011) Great ape cognition and captive care: Can cognitive challenges enhance well-being? Applied Animal Behaviour Science 135: 1-12.
- Clark FE (2013) Marine mammal cognition and captive care: A proposal for cognitive enrichment in zoos and aquariums. Journal of Zoo and Aquarium Research 1: 1-6.
- Puppe B, Ernst K, Schön PC, Manteuffel G (2007) Cognitive enrichment affects behavioural reactivity in domestic pigs. Applied Animal Behaviour Science 105: 75-86.
- Yamanashi Y, Hayashi M (2011) Assessing the effects of cognitive experiments on the welfare of captive chimpanzees (Pan troglodytes) by direct comparison of activity budget between wild and captive chimpanzees. Am J Primatol 73: 1231-1238. [Crossref]
- Hopper LM, Shender MA, Ross SR (2016) Behavioral research as physical enrichment for captive chimpanzees. Zoo biology 35: 293-297. [Crossref]
- Whitehouse J, Micheletta J, Powell LE, Bordier C, Waller BM (2013) The impact of cognitive testing on the welfare of group housed primates. PLoS One 8: e78308. [Crossref]
- Ruby S, Buchanan-Smith HM (2015) The effects of individual cubicle research on the social interactions and individual behavior of brown capuchin monkeys (Sapajus apella). Am J Primatol 77: 1097-1108. [Crossref]
- Skinner BF (1938) The Behavior of Organisms (Appleton-Century-Crofts, New York). Spaulding WD, Storms L, Goodrich, V, Sullivan M (1986) Applications of experimental psychopathology in psychiatric rehabilitation. Schizophrenia Bulletin 12: 560-577. [Crossref]
- Green DM (1966) Signal detection theory and psychophysics [by] David M Green [and] John A Swets. Wiley, New York.
- Stebbins WC (1970b) Principles of animal psychophysics. Animal psychophysics: The design and conduct of sensory experiments 12: 1-19.
- Fay RR (1988) Hearing in vertebrates: a psychophysics databook. Hill-Fay Associates Winnetka, IL.
- Gellermann, LW (1933) Chance orders of alternating stimuli in visual discrimination experiments. The Pedagogical Seminary and Journal of Genetic Psychology 42: 206-208.
- Davidson JE, Sternberg RJ (2003) The psychology of problem solving. Cambridge University Press.
- Stebbins WC (1970a) Animal Psychophysics: the design a conduct of sensory experiments. Plenum Press, New York and London.
- Keen HA, Nelson OL, Robbins CT, Evans M, Shepherdson DJ, et al. (2014) Validation of a novel cognitive bias task based on difference in quantity of reinforcement for assessing environmental enrichment. Animal cognition 17: 529-541. [Crossref]
- Langbein J, Siebert K, Nürnberg G (2009) On the use of an automated learning device by group-housed dwarf goats: Do goats seek cognitive challenges? Applied Animal Behaviour Science 120: 150-158.
- Defran RH, Pryor K (1980) The behavior and training of cetaceans in captivity. Cetacean behavior: Mechanisms and functions 319-362.
- Terry R (1986) The behaviour and trainability of Sotalia fluviatilis guianensis in captivity: a survey. Aquatic Mammals 12: 71-79.
- Heinrich B (1988) Why do ravens fear their food? The Condor 90: 950-952.
- Bradbury JW, Balsby TJ (2016) The functions of vocal learning in parrots. Behavioral Ecology and Sociobiology 70: 293-312.






