Abstract
Cryptosporidium parasites infecting horses are distributed worldwide. The reported prevalence of equine cryptosporidiosis is quite variable, ranging from 0% to 37.8%. According to the literature, horses can become infected with various Cryptosporidium species, including Cryptosporidium parvum, Cryptosporidium hominis, Cryptosporidium muris, Cryptosporidium horse genotype, Cryptosporidium tyzzeri, and Cryptosporidium andersoni. Here we review the literature on equine cryptosporidiosis dating back to 1978. Because of the possibility of the human parasite Cryptosporidium hominis infecting horses, we examined the phylogeny of the Cryptosporidium small subunit ribosomal RNA and GP60 genes isolated from horses and found evidence of equine cryptosporidiosis caused by this species which is commonly assumed to be restricted to humans.
Key words
coccidia, cryptosporidium spp., foal, horse
Introduction
Cryptosporidiosis is a globally distributed infection causing sporadic gastroenteritis, abdominal pain, vomiting and fever. Several zoonotic species have been identified in humans, but most cases of human cryptosporidiosis are caused by C. parvum and C. hominis [1]. Cryptosporidiosis has long been recognized as a serious veterinary problem in neonatal ruminants [2]. C. parvum is the main species found in goats [3], lambs, calves[4] and foals [5].
The observation that equines may be infected with Cryptosporidium species similar to those infecting humans [6-11], prompted to focus this review on the global occurrence of Cryptosporidium spp. in equines and review the literature describing Cryptosporidium species found in horses.
Taxonomy
The genus Cryptosporidium is classified in the phylum Apicomplexa, class Sporozoae, subclass Coccidia, order Eucoccidiida, suborder Eimeriina and family Cryptosporididae [12]. The taxonomic classification of the genus is still being debated, as recent phylogenetic studies have shown proximity to the subclass Cryptogregaria, which is part of the Gregarines in the phylum Apicomplexa, class Gregarinomorphea. This proposed classification is based on reports that oocysts can multiply outside the host, on the presence of a specialized feeding organelle and the absence of the apicoplast [13-19]. Recent studies using DNA sequencing have led to the currently proposed taxonomy of the genus Cryptosporidium comprising 31 species and more than 70 genotypes [20], including the newly described species Cryptosporidium proliferans [21] and Cryptosporidium avium [22]. The taxonomic classification of Cryptosporidium isolated from equines based exclusively on genetic data is uncertain, particularly in the absence of characteristic morphological traits of the oocysts and a lack of data on host range.
Epidemiology of equine cryptosporidiosis
Cryptosporidium in equines is distributed worldwide an on all continents. Most studies reviewed here are from American and European countries 38.4%, and 35.9% respectively, while studies from Asia, Oceania and Africa are 12.8%, 7.7% and 5.1%, respectively. Most studies reporting on Cryptosoporidium in equines are from North America, only three reports described equine infections from South Americans, whereas literature on Cryptosporidium in equines from Central America no was identified in our search (Table 1).
Table 1. Global occurrence of Cryptosporidium, species and GP60 genotype in equines according to diagnostic methodology
Continent |
Country |
Total Horses |
Positives |
Species |
GP60 Genotype |
Diagnostic Method |
Reference |
Africa |
Algeria |
138 |
4 |
Cryptosporidium hedgehog |
---------- |
PCR |
[52] |
Algeria |
219 |
5 |
Cryptosporidium parvum |
IIaA16G1R1 |
PCR |
[6] |
Cryptosporidium hominis |
IkA15G1 |
Cryptosporidium muris |
RN66 |
America |
Brazil |
396 |
3 |
Cryptosporidium spp. |
----------- |
CF/SMB |
[53] |
Brazil |
196 |
39 |
Cryptosporidium spp. |
---------- |
MKT |
[54] |
Brazil |
92 |
|
Cryptosporidium parvum |
IIaA18G3R1 |
PCR |
[11] |
20 |
Cryptosporidium parvum |
IIaA15G2R1 |
| |
Cryptosporidium hominis |
IkA20G1 |
Canada |
2 |
2 |
Cryptosporidium spp. |
------------ |
SF/EM |
[55] |
Canada |
35 |
8 |
Cryptosporidium spp. |
|
FS/IM |
[41] |
United States of America |
29 |
5 |
Cryptosporidium spp. |
------------ |
HE/W-G/EM |
[39] |
United States of America |
14 |
- |
------------------------ |
------------ |
SC |
[25] |
United States of America |
22 |
22 |
Cryptosporidium spp. |
------------ |
FF |
[56] |
United States of America |
91 |
-- |
------------------------ |
------------ |
DFA/LC |
[26] |
United States of America |
366 |
13 |
----------------------- |
------------ |
AF/IFA/FC |
[57] |
United States of America |
1 |
1 |
Cryptosporidium spp. |
------------ |
IM |
[58] |
United States of America |
223 |
3 |
Cryptosporidium spp. |
----------- |
MAF |
[59] |
United States of America |
349 |
16 |
Cryptosporidium horse genotype |
VIaA14G2 |
DFA/PCR |
[34] |
United States of America |
88 |
18 |
Cryptosporidium |
---------- |
RT- PCR |
[40] |
United States of America |
84 |
|
Cryptosporidium parvum |
IIaA13G2R2 |
PCR |
[60] |
28 |
Cryptosporidium parvum |
IIaA15G2R1 |
| |
Cryptosporidium parvum |
IIaA17G2R1 |
Asia |
Jordan |
74 |
6 |
Cryptosporidium parvum |
-------------- |
qPCR |
[61] |
China |
262 |
7 |
Cryptosporidium horse genotype |
VIaA15G4 |
PCR |
[36] |
China |
29 |
2 |
Cryptosporidium andersoni |
MLST |
SSFT/PCR |
[7] |
China |
--- |
5 |
Cryptosporidium parvum |
IIdA19G1 |
PCR |
[9] |
Cryptosporidium hominis |
IkA16G1 |
China |
333 |
|
Cryptosporidium andersoni |
IdA15 |
PCR |
[10] |
6 |
Cryptosporidium hominis |
Europe |
Czech Republic |
2 |
11 |
Cryptosporidium horse genotype |
VIaA11G3 |
PCR |
[23] |
Cryptosporidium parvum |
Czech Republic |
3 |
3 |
Cryptosporidium parvum |
---------- |
PCR |
[62] |
Czech Republic/Poland |
352 |
12 |
Cryptosporidium parvum |
IIaA15G2R1 |
ACMVSM, PCR |
[8] |
Cryptosporidium tyzzeri |
IXbA22R9 |
Cryptosporidium muris |
|
Cryptosporidium horse genotype |
VIaA15G4 |
Czech Republic/Poland |
352 |
12 |
Cryptosporidium parvum |
IIaA15G2R1 |
ACMVSM, PCR |
[8] |
Cryptosporidium tyzzeri |
IXbA22R9 |
Cryptosporidium muris |
|
Cryptosporidium horse genotype |
VIaA15G4 |
England |
644 |
17 |
Cryptosporidium spp. |
------------ |
IF |
[63] |
England |
80 |
40 |
Cryptosporidium parvum |
----------- |
EPM /ZN |
[64] |
England |
52 |
2 |
Cryptosporidium parvum |
---------- |
DI/EM/PCR |
[47] |
England and Wales |
18 |
6 |
Cryptosporidium parvum |
---------- |
IS/IM/PCR |
[65] |
Greece/Belgium/Netherlands/Germany |
398 |
8 |
Cryptosporidium horse genotype |
----------- |
IFA/PCR |
[37] |
Italy |
150 |
12 |
Cryptosporidium parvum |
---------- |
ZN/CF/LS/SF /DFA/PCR |
[66] |
Italy |
74 |
2 |
Cryptosporidium parvum |
---------- |
ELISA/PCR |
[67] |
Italy |
37 |
14 |
Cryptosporidium parvum |
VIaA15G4 |
PCR |
[35] |
Cryptosporidium horse genotype |
VIaA15G4 |
Italy |
73 |
14 |
Cryptosporidium parvum |
IIdA21G0 |
ZN/PCR |
[27] |
Cryptosporidium parvum |
IIdA22G1 |
Cryptosporidium horse genotype |
VIaA15G4 |
Cryptosporidium parvum |
IIaA23GR1 |
Italy |
64 |
2 |
Cryptosporidium parvum |
IIdA23G1 |
ZN/PCR |
[44] |
Switzerland |
1 |
1 |
Cryptosporidium parvum |
----------- |
ZN/F/S/PCR |
[42] |
Oceania |
New Zealand |
17 |
5 |
Cryptosporidium parvum |
---------- |
HE/PAS/G/AF/PCR |
[28] |
New Zealand |
9 |
9 |
Cryptosporidium parvum |
IIaA18G3R1 |
PCR |
[5] |
New Zealand |
131 |
67 |
Cryptosporidium parvum |
---------- |
PCR |
[38] |
Subtitle: Tissues Stained with Hematoxylin and Eosin (HE), Wolbach-Giemsa (W-G), Electronic Microscopy (EM), Sucrose Centrifugation (SC), Sucrose Flotation (SF), Fecal Flotation (FF), Immunoflorescence (IF), Direct Fluorescent Antibody (DFA), Levitation Centrifugation Tests (LC), Sheather Fotation (SF), Immunofluorescent Microscopy (IM), Acid-Fast (AF), Immunofluorescence Test (IFA), Flow Cytometry (FC), Polymerase Chain Reaction (PCR), Epifluorescence Microscopy (EPM), Ziehl-Neelsen (ZN), Periodic Acid-Schiff (PAS), Giemsa (G), Direct Immunofluorescence (DI), Flotation (F), Sedimentation (S), Centrifugation and Flotation (CF), Safranine–Methylene-Blue (SMB), Modified Acid-Fast (MAF), Lugol Staining (LS), Imunogenetic Separation (IS), Enzyme-Linked Immunosorbent Assay (ELISA), Modified Kinyoun Technique (MKT), Real-time-PCR – RT-PCR, Aniline-Carbol-Methyl Violet Staining Method (ACMVSM), Sheather's Sugar Floatation Technique (SSFT
Between 2003 and today, many researchers have used PCR to identify Cryptosporidium species in equine samples, advancing our understanding of the occurrence of this parasite and the role of horses and foals in the epidemiology of cryptosporidiosis. Table 1 shows that horses can become infected with C. parvum, C. hominis, C. muris, C. tyzzeri, C. andersoni, C. erinacei and what has been named Cryptosporidium horse genotype [23]. The first two species are infectious to humans, and are responsible for approximately 90% of human cryptosporidiosis cases [1,24]. The susceptibility of horses to the same species infecting humans indicates that horses may play a role in the zoonotic transmission of these parasites.
It is important to note that the reported prevalence of Cryptosporidium in equines samples is quite variable, ranging from 0% [25,26] to 37.8% [27]. In our literature survey, equines of different age groups, genders and breeds were included, which may account for the wide range in reported prevalence. Thus, in Table 1, the actual prevalence may differ, in part due to the use of different diagnostic techniques, which makes it difficult to compare results from different surveys.
In New Zealand, a first-ever study was conducted on an outbreak of cryptosporidiosis in nine purebreed foals. Epidemiological, clinical, pathological and genetic data were compiled, and C. parvum isolates identified based on restriction fragment polymorphism and on the sequence of the 18S rRNA gene as belonging to what was then named “cattle” genotype of C. parvum [28] and later renamed C. parvum.
The first description of the infection of equines by Cryptosporidium hedgehog genotype (renamed C. erinacei [29], is from Algeria [30]. Cryptosporidium DNA belonging to this species was identified using PCR targeting the 18S rRNA gene and the gp60 gene. This observation indicates that the horses may participate as hosts or mechanical carriers in C. erinacei life cycle [30]. C. hominis DNA was amplified from an equine fecal sample from Algeria using PCR targeting the Heat shock Protein 70 (HSP70), the Cryptosporidium Oocyst Wall Protein (COWP) and TSP-related adhesive protein of Cryptosporidium-1(TRAP-C1) genes. C. muris was described for the first time in equine samples from Algeria by sequencing the 18S rRNA and GP60 genes [6]. These findings reinforces the hypothesis of a new species of Cryptosporidium infecting equines because until recently, few species of Cryptosporidium were found in horses, however, with the reports of C. muris and C. tyzzeri increase susceptibility to infection in horses [8]. As there are a few reports of equine infected with C. hominis [6,10,11], Laatamna’s report of a horse in Algeria infected with this species is intriguing, due to the fact C. hominis is usually described infecting humans. Similar observations were reported from China [9] and from Brazil [11]. Interestingly, these studies found similar GP60 genotypes, designated IkA15G1, IkA16G1 and IkA20G1, respectively. Although the GP60 genotypes identified in these studies are not identical, the genotypes of additional genetic markers, such as the 18S rRNA, actin and HSP70, are consistent with horses being susceptible to C. hominis or a genotypically similar species (Figure 1). Although a human case of cryptosporidiosis caused by an isolate with GP60 genotype Ik was apparently identified (NCBI nucleotide accession number KU727290), Ik alleles are not frequently observed in humans. On the other hand, similar GP60 alleles were found in C. cuniculus [31], a species infecting rabbits and humans which is closely related to C. hominis. The significance of equine infections with C. hominis, or C. hominis-like parasites, for zoonotic transmission of this species is unknown.
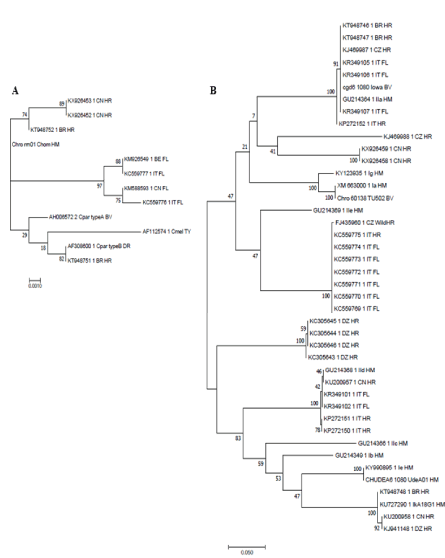
Figure 1. Phylogenetic trees based on the partial sequence of the 18S rRNA and GP60 genes show the phylogenetic context of Cryptosporidium sequences isolated from horses. A) Trees were generated using a 583-nt fragment of the 18S gene from equine Cryptosporidium isolates and C. hominis, C. parvum and C. meleagridis reference sequences. B) Phylogenetic tree based on approximately 100-nt fragment of the GP60 gene. Scale bars indicate 0.0010 and 0.050, respectively. BV, bovine, HM, human; DY donkey; HR/FL/WildHR, equine (horse, foal, wild horse, respectively
The occurrence of Cryptosporidium spp. is often associated with urban areas and particularly with the contamination of water supplies with human fecal material [32,33]. In Brazil, C. hominis GP60 genotype IkA20G1 and C. parvum genotypes IIaA18G3R1 and IIaA15G2R1 were collected from foals that drank water from a river that receives untreated urban wastewater [11]. The putative presence of C. hominis in horses raises public health concerns [10], since is distributed in all continents of the world. The identification in recent studies of C. hominis, or C. hominis-like parasites, in horses [6,10,11] suggests that contact between humans and horses may favor the transmission of this species, which is not commonly associated with zoonotic transmission.
In the Czech Republic, equines infected with C. parvum and with the horse genotype were identified using PCR targeting the 18S rDNA and HSP-70 genetic markers. This work showed that the horse genotype is more closely related to Cryptosporidium wrairi, there being a 98.7% similarity for 18S and 99% similarity for HSP-70 [23] between these taxa. C. parvum and the Cryptosporidium horse genotype have been reported in several other studies from different countries, such as the Czech Republic [8], the USA [34], Italy [27,35], China [36], and Belgium [37]. Based on these observations, we conclude that horses worldwide may be infected with Cryptosporidium parasites. Published evidence suggests that C. parvum/Cryptosporidium horse genotype and C. hominis, are the most common agent of equine infections followed by C. andersoni and C. muris, C. hedgehog (erinacei) and C. tyzzeri.
PCR allowed Cryptosporidium species detection in equine population, including zoonotic ones. These findings are important and have generated worries for public health.
Pathogenesis and clinical manifestations
The incidence of clinical C. parvum infections in newborn foals may be underestimated, perhaps accounting for cases empirically diagnosed as foal heat diarrhea [38]. In one of the earliest reports on equine cryptosporidiosis, the susceptibility of immunodeficient foals to this infection was described [39]. Immunocompetent foals where also found to develop diarrhea while eliminating Cryptosporidium oocysts [25], potentially causing economic loss to owners. Thus, it is often unclear whether the primary cause of symptoms is cryptosporidiosis or a viral agent. In most cases, other infectious agents that can also causes diarrhea were not considered or not diagnosed. In Kentucky, Cryptosporidium infections are most commonly observed in foals affected by other infections, caused by bacteria and viruses [25,40]. Symptoms are poorly described in equines [41]. More researches need to be performed to elucidate equine cryptosporidiosis symptomatology and pathogenicity of Cryptosporidium species.
In New Zealand, in post-mortem examinations of three foals, it was found that their intestines were full of fluid, were dilated, swollen and thin-walled, with no inflammation in the abdominal cavity. Microscopic examination of hematoxylin eosin stained sections of intestinal tissue revealed the presence in epithelial cells of numerous round organisms approximately two to five microns in size in much of the duodenum. These lesions were found to be consistent with cryptosporidiosis [28]. In Switzerland, a 9-day-old foal with diarrhea, fever and feces with fetid odor was also found to be infected with C. parvum [42].
Cryptosporidium caused inflammation and atrophy of the intestinal microvillous region with loss of absorptive surface, imbalance in the transport of nutrients and impairment in animal productivity [43].
Zoonotic potential
Currently, there is only one description of zoonotic transmission in equines. A study conducted in Italy it was shown that six veterinary students and hospitalized foals had symptoms consistent with cryptosporidiosis. C. parvum with GP60 genotype IIdA23G1 [44] was found in humans and animals. Students could have been infected by being in contact with foals infected with Cryptosporidium and because oocysts are highly resistant to environmental conditions and disinfectants, remaining viable for a long period of time [45].
An observation consitent with transmission between horses and other livestock species is a report of a horse infected with C. andersoni, a species commonly found in the abomasum of adult ruminants. The possible transmission between cattle and horses has also been reported from China [7]. Further evidence of horses being susceptible to C. parvum was reported from the UK, confirming that equines can potentially be a source of zoonotic infection of humans [46,47]. The extent of genetic diversity of Cryptosporidium isolated from equines was studied by sequencing polymorphic regions of the GP60 glycoprotein and heat shock protein HSP70 gene. These analyses revealed species and genotypes of Cryptosporidium genetically closely resembling those found in humans and bovines [5].
Cryptosporidium horse genotype was initially described in Przewalski’s wild horse foal [23], being considered as specific genotype of horses, as found in New York, in foals and their mares has been reported [34]. However, the Cryptosporidium horse genotype was also found in an immuno-compromised woman in England [48], suggesting a risk to human health.
Many Cryptosporidium species were found in farm animals samples [41,49]. Generally, these animals drink untreated water from rivers passing through the farm and wells. This water may be contaminated with oocysts of zoonotic and zooantroponotic species of Cryptosporidium. Once they ingest oocysts, animals can be infected or act as mechanical carriers, shedding Cryptosporidium oocysts and contaminating pasture. Most of farm animals are herbivors, so there are two ways they can be infected: drinking contaminated water or eating contaminated grass. Oocysts in the environment can be also carried by rain to watercourses allowing another susceptible living being infection.
C. parvum has been found in faecal samples of livestock [32,50]. C. parvum was also found in faecal samples of wild mustangs and Chincoteague ponies. These animals have minimal contact with humans; however, they ingest water and graze in the same places as cattle and wildlife such as deer and elk [51].
Conclusion
We verified that the genus Cryptosporidium with its species is distributed worldwide in the equine population. We also observed that most pathogenic species for humans detected in equines are C. parvum and C. hominis, evidencing a public health problem.
Competing interest
The authors declare that they have no competing interests.
References
- 1.Cacciò SM, Putignani L (2014) Epidemiology of human cryptosporidiosis, in Cryptosporidium: Parasite and Disease, Cacciò SM, Widmer G, Eds, Springer, New York.
- Tzipori S (1983) Cryptosporidiosis in animals and humans.Microbiol Rev47: 84-96. [Crossref]
- 3.Robertson LJ (2009) Giardia and Cryptosporidium infections in sheep and goats: A review of the potential for transmission to humans via environmental contamination. Epidemiol Infect 137: 913–921.
- Santín M, Trout JM, Fayer R (2008) A longitudinal study of cryptosporidiosis in dairy cattle from birth to 2 years of age.Vet Parasitol155: 15-23. [Crossref]
- Grinberg A, Learmonth J, Kwan E, Pomroy W, Lopez Villalobos N, Gibson I, et al. (2008) Genetic diversity and zoonotic potential of Cryptosporidium parvum causing foal diarrhea. J Clin Microbiol 46: 2396-2398.
- Laatamna AE, Wagnerová P, Sak B, Kvetonová D, Xiao L, et al. (2015) Microsporidia and Cryptosporidium in horses and donkeys in Algeria: Detection of a novel Cryptosporidium hominis subtype family (Ik) in a horse. Vet Parasitol 208: 135-142.
- Liu A, Zhang J, Zhao J, Zhao W, Wang R, et al. (2015) The first report of Cryptosporidium andersoni in horses with diarrhea and multilocus subtype analysis. Parasit Vectors 8: 483.
- Wagnerová P, Sak B, McEvoy J, Rost M, Perec M, et al. (2015) Genetic diversity of Cryptosporidium spp. including novel identification of the Cryptosporidium muris and Cryptosporidium tyzzeri in horses in the Czech Republic and Poland. Parasitol Res 114: 1619-1624. [Crossref]
- Jian F, Liu A, Wang R, Zhang S, Qi M, et al. (2016) Common occurrence of Cryptosporidium hominis in horses and donkeys. Infect Genet Evol 43: 261-266. [Crossref]
- Deng L, Li W, Zhong Z, Gong C, Cao X, et al. (2017) Occurrence and Genetic Characteristics of Cryptosporidium hominis and Cryptosporidium andersoni in Horses from Southwestern China. J Eukaryot Microbiol 64: 716-720. [Crossref]
- Inácio SV, Widmer G, Brito RLL, Zucatto AS, de Aquino MC, et al. (2017) First description of Cryptosporidium hominis GP60 genotype IkA20G1 and Cryptosporidium parvum GP60 genotypes IIaA18G3R1 and IIaA15G2R1 in foals in Brazil. Vet Parasitol 233: 48-51. [Crossref]
- 12.Plutzer J, Karanis P (2009) Genetic polymorphism in Cryptosporidium species: an update.Vet Parasitol165: 187-199. [Crossref]
- Koh W, Clode PL, Monis P, Thompson RA (2013) Multiplication of the waterborne pathogen Cryptosporidium parvum in an aquatic biofilm system. Parasit Vectors 6: 1. [Crossref]
- Koh W, Thompson A, Edwards H, Monis P, Clode PL (2014) Extracellular excystation and development of Cryptosporidium: Tracing the fate of oocysts within Pseudomonas aquatic biofilm systems. BMC Microbiol 14: 1-12.
- Cavalier-Smith T (2014) Gregarine site-heterogeneous 18S rDNA trees, revision of gregarine higher classification, and the evolutionary diversification of Sporozoa. Eur J Protistol 50: 472-495. [Crossref]
- Huang L, Zhu H, Zhang S, Wang R, Liu L, et al. (2014) An in vitro model of infection of chicken embryos by Cryptosporidium baileyi.Exp Parasitol147: 41-47. [Crossref]
- Clode PL, Koh WH, Thompson RCA (2015) Life without a Host Cell: What is Cryptosporidium?Trends Parasitol31: 614-624. [Crossref]
- Aldeyarbi HM, Karanis P (2016) The Ultra-Structural Similarities between Cryptosporidium parvum and the Gregarines. J Eukaryot Microbiol 63: 79-85. [Crossref]
- Ryan U, Paparini A, Monis P, Hijjawi N (2016) It's official - Cryptosporidium is a gregarine: What are the implications for the water industry?Water Res105: 305-313. [Crossref]
- Ryan U, Hijjawi N (2015) New developments in Cryptosporidium research.Int J Parasitol45: 367-373. [Crossref]
- Kvác M, Havrdová N, Hlásková L, Danková T, Kandera J, et al. (2016) Cryptosporidium proliferans n. sp. (Apicomplexa: Cryptosporidiidae): Molecular and Biological Evidence of Cryptic Species within Gastric Cryptosporidium of Mammals. PLoS One 11: 1-24.
- Holubová N, Sak B, Horčičková M, Hlásková L, Květoňová D, et al. (2016) Cryptosporidium avium n. sp. (Apicomplexa: Cryptosporidiidae) in birds.Parasitol Res115: 2243-2251. [Crossref]
- Ryan U, Xiao L, Read C, Zhou L, Lal A, et al. (2003) Identification of Novel Cryptosporidium Genotypes from the Czech Republic. Appl Environ Microbiol 69: 4302-4307. [Crossref]
- Leoni F, Amar C, Nichols G, Pedraza-Díaz S, McLauchlin J (2006) Genetic analysis of Cryptosporidium from 2414 humans with diarrhoea in England between 1985 and 2000.J Med Microbiol55: 703-707. [Crossref]
- Reinemeyer CR, Kline RC, Stauffer GD (1984) Absence of cryptosporidium oocysts in faeces of neonatal foals.Equine Vet J16: 217-218. [Crossref]
- Johnson E, Atwill ER, Filkins ME, Kalush J (1997) The prevalence of shedding of Cryptosporidium and Giardia spp. based on a single fecal sample collection from each of 91 horses used for backcountry recreation. J Vet. Diagnostic Investig 9: 56-60. [Crossref]
- Galuppi R, Piva S, Castagnetti C, Iacono E, Tanel S, et al. (2015) Epidemiological survey on Cryptosporidium in an Equine Perinatology Unit.Vet Parasitol210: 10-18. [Crossref]
- Grinberg A, Oliver L, Learmonth JJ, Leyland M, Roe W, et al. (2003) Identification of Cryptosporidium parvum “cattle” genotype from a severe outbreak of neonatal foal diarrhoea. Vet Rec 153: 628-631. [Crossref]
- Kváč M, Hofmannová L, Hlásková L, Květoňová D, Vítovec J,et al. (2014) Cryptosporidium erinacei n. sp. (Apicomplexa: Cryptosporidiidae) in hedgehogs.Vet Parasitol201: 9-17. [Crossref]
- Laatamna AE, Wagnerová P, Sak B, Kvetonová D, Aissi M, et al. (2013) Equine cryptosporidial infection associated with Cryptosporidium hedgehog genotype in Algeria. Vet Parasitol 197: 350-353. [Crossref]
- Robinson G, Wright S, Elwin K, Hadfield SJ, Katzer F, et al. (2010) Re-description of Cryptosporidium cuniculus Inman and Takeuchi, 1979 (Apicomplexa: Cryptosporidiidae): Morphology, biology and phylogeny. Int J Parasitol 40: 1539-1548. [Crossref]
- Xiao L, Feng Y (2008) Zoonotic cryptosporidiosis.FEMS Immunol Med Microbiol52: 309-323. [Crossref]
- Chako CZ, Tyler JW, Schultz LG, Chiguma L, Beerntsen BT (2010) Cryptosporidiosis in people: it's not just about the cows.J Vet Intern Med24: 37-43. [Crossref]
- Burton AJ, Nydam DV, Dearen TK, Mitchell K, Bowman DD, et al. (2010) The prevalence of Cryptosporidium, and identification of the Cryptosporidium horse genotype in foals in New York State.Vet Parasitol174: 139-144. [Crossref]
- Caffara M, Piva S, Pallaver F, Iacono E, Galuppi R (2013) Molecular characterization of Cryptosporidium spp. from foals in Italy.Vet J198: 531-533. [Crossref]
- Qi M, Zhou H, Wang H, Wang R, Xiao L, et al. (2015) Molecular identification of Cryptosporidium spp. and Giardia duodenalis in grazing horses from Xinjiang, China.Vet Parasitol209: 169-172. [Crossref]
- Kostopoulou D, Casaert S, Tzanidakis N, Doorn D, van Demeler J, et al. (2015) The occurrence and genetic characterization of Cryptosporidium and Giardia species in foals in Belgium, The Netherlands, Germany and Greece. Vet Parasitol 211: 170-174. [Crossref]
- Grinberg A, Pomroy WE, Carslake HB, Shi Y, Gibson IR, et al. (2009) A study of neonatal cryptosporidiosis of foals in New Zealand.N Z Vet J57: 284-289. [Crossref]
- Snyder SP, England JJ, McChesney AE (1978) Cryptosporidiosis in immunodeficient Arabian foals.Vet Pathol15: 12-17. [Crossref]
- Slovis NM, Elam J, Estrada M, Leutenegger CM (2014) Infectious agents associated with diarrhoea in neonatal foals in central Kentucky: A comprehensive molecular study. Equine Vet J 46: 311-316.
- Olson ME, Thorlakson CL, Deselliers L, Morck DW, McAllister TA (1997) Giardia and Cryptosporidium in Canadian farm animals.Vet Parasitol68: 375-381. [Crossref]
- Imhasly A, Frey CF, Mathis A, Straub R, Gerber V (2009) [Cryptosporidiose (C. parvum) in a foal with diarrhea].Schweiz Arch Tierheilkd151: 21-26. [Crossref]
- Thompson RC, Palmer CS, O'Handley R (2008) The public health and clinical significance of Giardia and Cryptosporidium in domestic animals.Vet J177: 18-25. [Crossref]
- Galuppi R, Piva S, Castagnetti C, Sarli G, Iacono E, et al. (2016) Cryptosporidium parvum: From foal to veterinary students. Vet Parasitol 219: 53–56.
- Silverlås C, Björkman C, Egenvall A (2009) Systematic review and meta-analyses of the effects of halofuginone against calf cryptosporidiosis.Prev Vet Med91: 73-84. [Crossref]
- Chalmers RM, Grinberg A (2005) Significance of Cryptosporidium parvum in horses.Vet Rec156: 688. [Crossref]
- Chalmers RM, Thomas AL, Butler BA, Morel MC (2005) Identification of Cryptosporidium parvum genotype 2 in domestic horses.Vet Rec156: 49-50. [Crossref]
- Robinson G, Elwin K, Chalmers RM (2008) Unusual Cryptosporidium genotypes in human cases of diarrhea. Emerg Infect Dis 14: 1800-1802. [Crossref]
- Niine T, Dorbek-Kolin E, Lassen B, Orro T (2018) Cryptosporidium outbreak in calves on a large dairy farm: Effect of treatment and the association with the inflammatory response and short-term weight gain. Res Vet Sci 117: 200-208. [Crossref]
- Ng JSY, Eastwood K, Walker B, Durrheim DN, Massey PD, et al. (2012) Evidence of Cryptosporidium transmission between cattle and humans in northern New South Wales. Exp Parasitol 130: 437-441. [Crossref]
- Wagnerová P, Sak B, McEvoy J, Rost M, Sherwood D, et al. (2016) Cryptosporidium parvum and Enterocytozoon bieneusi in American Mustangs and Chincoteague ponies. Exp Parasitol 162: 24-27. [Crossref]
- Laatamna AE, Wagnerová P, Sak B, Kvetonová D, Aissi M, et al. (2013) Equine cryptosporidial infection associated with Cryptosporidium hedgehog genotype in Algeria. Vet Parasitol 197: 350-353. [Crossref]
- Souza PNB De, Bomfim TCB, Huber F, Abboud LCS, Gomes RS (2009) Natural infection by Cryptosporidium sp., Giardia sp. and Eimeria leuckarti in three groups of equines with different handlings in Rio de Janeiro, Brazil. Vet Parasitol 160: 327-333. [Crossref]
- Inácio SV, de Brito RL, Zucatto AS, Coelho WM, de Aquino MC, et al. (2012) Cryptosporidium spp. infection in mares and foals of the northwest region of São Paulo State, Brazil.Rev Bras Parasitol Vet21: 355-358. [Crossref]
- Gajadhar AA, Caron JP, Allen JR (1985) Cryptosporidiosis in two foals.Can Vet J26: 132-134. [Crossref]
- Coleman SU, Klei TR, French DD, Chapman MR, Corstvet RE (1989) Prevalence of Cryptosporidium sp in equids in Louisiana.Am J Vet Res50: 575-577. [Crossref]
- Cole DJ, Snowden K, Cohen ND, Smith R (1998) Detection of Cryptosporidium parvum in horses: Thresholds of acid-fast stain, immunofluorescence assay, and flow cytometry. J Clin Microbiol 37: 457-460. [Crossref]
- McKenzie DM, Diffay BC (2000) Diarrhoea associated with cryptosporidial oocyst shedding in a quarterhorse stallion.Aust Vet J78: 27-28. [Crossref]
- Frederick J, Giguère S, Sanchez LC (2009) Infectious agents detected in the feces of diarrheic foals: a retrospective study of 233 cases (2003-2008).J Vet Intern Med23: 1254-1260. [Crossref]
- Wagnerová P, Sak B, McEvoy J, Rost M, Sherwood D, et al. (2016) Cryptosporidium parvum and Enterocytozoon bieneusi in American Mustangs and Chincoteague ponies. Exp Parasitol 162: 24-27. [Crossref]
- Hijjawi N, Mukbel R, Yang R, Ryan U (2016) Genetic characterization of Cryptosporidium in animal and human isolates from Jordan.Vet Parasitol228: 116-120. [Crossref]
- Hajdusek O, Ditrich O, Slapeta J (2004) Molecular identification of Cryptosporidium spp. in animal and human hosts from the Czech Republic.Vet Parasitol122: 183-192. [Crossref]
- Netherwood T, Wood JL, Townsend HG, Mumford JÁ, Chanter N (1996) Foal diarrhoea between 1991 and 1994 in the United Kingdom associated with Clostridium perfringens, rotavirus, Strongyloides westeri and Cryptosporidium spp. Epidemiol Infect 117: 375-83. [Crossref]
- Sturdee AP, Bodley-Tickell AT, Archer A, Chalmers RM (2003) Long-term study of Cryptosporidium prevalence on a lowland farm in the United Kingdom.Vet Parasitol116: 97-113. [Crossref]
- Smith RP, Chalmers RM, Mueller-Doblies D, Clifton-Hadley FA, Elwin K, et al. (2010) Investigation of farms linked to human patients with cryptosporidiosis in England and Wales. Prev Vet Med 94: 9-17. [Crossref]
- Veronesi F, Passamonti F, Cacciò S, Diaferia M, Piergili Fioretti D (2010) Epidemiological survey on equine Cryptosporidium and Giardia infections in Italy and molecular characterization of isolates. Zoonoses Public Health 57: 510-517. [Crossref]
- Perrucci S, Buggiani C, Sgorbini M, Cerchiai I, Otranto D, et al. (2011) Cryptosporidium parvum infection in a mare and her foal with foal heat diarrhoea.Vet Parasitol182: 333-336. [Crossref]

