Abstract
Objective: To evaluate the inhibitory effects on DPP-IV of six antidiabetic Chinese patent medicines and their formula contains of 33 Chinese medicines and 28 TCM monomeric compounds, so as to provides a reference for the study of its hypoglycemic mechanism.
Methods: Using a high-throughput screening (HTS) method for DPP-IV inhibitor with glycyl-prolyl-N-butyl-4-amino-1,8-naphthalimide (GP-BAN) as the probe substrate, human mixed plasma is the DPP-IV enzyme source. Using the efficient screening and evaluation method of DPP-IV inhibitors in the established complex system, the generation rate of GP-BAN metabolite BAN was measured by a microplate reader to evaluate its inhibitory effect.
Results: Coptidis Rhizoma and Cortex Moutan have the strong inhibitory effect on DPP-IV at 1 mg/mL, and the residual activity of DPP-IV was 27.90% and 35.44%, respectively. The DPP-IV inhibitory effect of the main chemical constituents in Coptidis Rhizoma and Cortex Moutan was further evaluated, 5 kinds of Coptidis Rhizoma monomer components wogonin, sanguinarine, Oxysanguinarine, jatrorrhizine and palmatine, 2 kinds of Cortex Moutan monomer components kaempferol and quercetin have strong inhibitory effects on DPP-IV, among which kaempferol has the best inhibitory effect on DPP-IV with IC50 of 9.26 μM. Further studies on inhibition kinetics and molecular docking showed that kaempferol is an effective non-competitive inhibitor of DPP-IV.
Conclusion: Six antidiabetic Chinese patent medicines have active components to inhibit the activity of DPP-IV.
Keywords
Dipeptidyl peptidase-IV; Type 2 diabetes; Coptis Chinensis; Cortex Moutan; Kaempferol
Introduction
Diabetes is a group of metabolic diseases characterized by hyperglycemia, which poses a threat to human public health, and its incidence is tending to be at a younger age [1]. Type 2 diabetes mellitus (T2DM) accounts for 90% of the total number of diabetes patients [2]. People with T2DM usually don't lose their ability to produce insulin completely, and some patients even produce too much, but insulin doesn't work as well. Therefore, many patients are relatively short of insulin and stimulating the secretion of insulin through some oral drugs is an important hypoglycemic therapy [3-5].
Dipeptidyl peptidase-IV (DPP-IV, CD26, EC3.4.14.5) is a type II transmembrane serine protease existing in the form of dimer, which is widely distributed in epithelial cells and vascular endothelial cells of mammalian organs, with the highest expression in kidney, and also as a soluble form in the plasma and other body fluids [6-8]. DPP-IV can specifically catalyze the hydrolysis and cleavage of the peptide bond of the amino acid residue proline (Pro) or alanine (Ala) at the 2nd position of the N-terminal of the polypeptide chain, such as incretin, neuropeptide, gastrin-releasing peptide, etc [9,10]. As an important peptide metabolism enzyme, DPP-IV has been the focus of attention in the pharmaceutical field because of its metabolic inactivation of endogenous endogenous glucagon-like peptide-1 (GLP-1). GLP-1 is an intestinal hormone that is highly elevated in plasma after food intake. By activating GLP-1 receptor, GLP-1 can promote insulin secretion in a glucose-dependent manner, inhibit glucagon secretion, delay gastric emptation, and reduce food intake through central appetite suppression, thus achieving the hypoglycemic effect. However, GLP-1 is rapidly degraded and inactivated by circulating DPP-IV, limiting its duration of action. Inhibiting the activity of DPP-IV could increase the concentration of GLP-1 in the body and prolong its action time. Thus, DPP-IV has become an important target for FDA-approved T2DM therapy [11,12]. Most of the DPP-IV inhibitors reported so far are synthetic compounds, and a number of DPP-IV inhibitors are marketed as treatment drugs for T2DM, such as Sitagliptin, Vildagliptin, and Saxagliptin [13-16], but natural DPP-IV inhibitors are less reported.
In recent years, Chinese patent medicine have played an increasingly important role in the field of glucose regulation abnormalities as well as diabetes treatment, and have the characteristics of holistic treatment, safety, and convenience in the process of clinical application [17,18], such as Liuwei Dihuang Pill [19,20], widely used in kidney-yin deficiency syndrome, Jiangtangjia Tablet and Xiaokeling Tablet for Qi-Yin deficiency type diabetes mellitus [21]. Jiangtangning capsules are used to reduce blood sugar and lipids to enhance glucose tolerance, Ganlu Xiaoke capsules are used to nourish yin and kidney, strengthen spleen and promote body fluid, and Yuquan Pills are used to regulate glucose and lipid metabolism and inflammation [22,23]. Nevertheless, the glucose-lowering mechanism of most of the glucose-lowering proprietary Chinese patent medicines is still unclear. Based on the enzymatic catalytic properties of DPP-IV, our team designed and developed a new high-specificity fluorescent probe substrate glycyl-prolyl-N-butyl-4-amino-1,8 -Naphthalene dicarboximide (GP-BAN), and based on this probe, a high-throughput screening platform for DPP-IV inhibitors using blood as the enzyme source was constructed [24,25]. In this study, the above-mentioned DPP-IV inhibitor screening platform was used to evaluate the DPP-IV inhibitory effect of six kinds of Chinese patent medicines. In-depth study of these Chinese patent medicines, their formula contains of Chinese medicines (33 pecies) and chemical components (28) were collected to explore the inhibitory effect on DPP-IV.
Materials and Methods
Chinese patent medicines
Jiangtangjia Tablet (batch No. 2003001013) was purchased from Jilin Aodong Yanbian Pharmaceutical Co., Ltd; Ganlu Xiaoke Capsule (batch No. 200204) was purchased from Zhongmei Huayi (Hebei) Pharmaceutical Co., Ltd; Yuquan Pill (batch No. 201001) was purchased from Chengdu Jiuzhitang Jinding Pharmaceutical Co., Ltd; Xiaokeling tablet (batch No. zja2001) was purchased from Yunnan Baiyao Group Co., Ltd; Liuwei Dihuang Pill (batch No. 20073147) was purchased from the pharmaceutical factory of Beijing Tongrentang Technology Development Co., Ltd; Jiangtangning capsule (batch No. 20200301) was purchased from Jilin gilji Pharmaceutical Co., Ltd.
Chinese medicines
Rhizoma Dioscoreae, Poria Cocos, Alisma Orientalis, Linn Gypsum, Astragalus Membranaceus, Ophiopogon Japonicus, Trichosanthis Radix, Lycii Fructus, Ginseng Radix et Rhizoma, Puerarialobata, Rehmannia Glutinosa, Radix Rehmanniae Preparata, Fructus Schisandrae Chinensis, Cortex Moutan, Rhizoma Anemarrhenae, Rhizoma Coptidis, Radix Scrophulariae, Radix Asparagi, Radix Glycyrrhizae, Rhizoma Atractylodis Macrocephalae, Stigma Maydis, Rhizoma Polygonati, Radix Pseudostellariae, Cortex Lycii, Fructus Corni, Radix Codonopsis, Semen Cuscutae, Radix Angelicae Sinensis, Ootheca Mantidis, Radix Ginseng Rubra, Wine Cornus, Rhizoma Chuanxiong, Rhizoma Polygonati Odorati. A total of 33 Chinese medicines Granules were purchased from Jiangyin Tianjiang Pharmaceutical Co,Ltd.
Chinese medicine monomer
Berberine, Oleanolic acid, Chlorogenic acid, Pharmacogenine, Jatrorrhizine, Wogonin, (+)Catechin, Protocatechuic acid, Tetrandrine, Paeoniflorin, Oxysanguinarine, β-sitosterol, 3-phenylpropionic acid, Kaempferol, Vanillic acid, Betulinic acid, Paeonol, Protocatechuic aldehyde, Peony bark glycoside C, Palmatine, Magnoflorine, Benzoyl paeoniflorin, Paeonol glucoside, Gentianic acid were purchased from Dalian Meilun Biotechnology Co,Ltd.; Quercetin, Wogonin, Coptisine hydrochloride were purchased from Chengdu Pfeiffer Biotechnology Co,Ltd. Gallic acid was purchased from Baoji Herbst Biotech Co., Ltd. Ferulic acid was purchased from Shanghai Titan Technology Co., Ltd. The purity of these compounds was >98%.
Reagents
Chromatographic grade DMSO was purchased from TEDIA (USA) as stock solutions for dissolving all compounds, GP-BAN was synthesized according to a previously published article [26], diluted with chromatographic grade DMSO when used. Chromatographic-grade acetonitrile was purchased from TEDIA (USA) in 100 mM phosphate-buffered saline, pH 7.4, in Millipore ultrapure water. Human plasma was purchased from Research Institute for Liver Diseases (Shanghai) Co.Ltd. Sitagliptin was purchased from Beijing Bailingwei Technology Co., Ltd. Electronic analytical balance (Germany Sartorius Co, Ltd.), KQ-800DE CNC ultrasonic cleaner (Kunshan Ultrasonic Instrument Co, Ltd.), Milli-Q ultra-pure Water machine (Shanghai Murray Technology Co, Ltd.), MS-100 constant temperature mixer (Hangzhou Aosheng Instrument Co, Ltd.); Multifuge X1R high-speed refrigerated centrifuge (American Thermo Fisher Scientific Co, Ltd.); Electronic balance (BSA224S, Max 220 g, d = 0.1 mg, Sartorius Scientific Instruments (Beijing) Co, Ltd.).
Preparation of the test sample solution
The Chinese patent medicines and Chinese medicines Granules were dissolved in ultrapure water in 1.5 mL EP tubes, extracted by ultrasonication (power 100 W, 40 kHz) for 30 min, prepared in gradients of 100 mg/mL, 10 mg/mL and 1 mg/mL and stored in a refrigerator at -20 ℃. 28 Chinese medicine monomers were prepared with DMSO in gradients of 50 mM, 5 mM and 0.5 mM. Sitagliptin solution (0.5 μM, 5 μM, 50 μM) was used for the positive control group.
High-throughput screening method for DPP-IV inhibitor
GP-BAN was used as a substrate to evaluate the inhibitory effects of Chinese patent medicines/ Chinese Medicine Monomer on DPP-IV and sitagliptin was used as a positive inhibitor [27]. The details for DPP-IV inhibition have been reported previously [24,28]. The incubation system for DPP-IV inhibitor screening was 200 μL. Firstly, PBS (pH 7.4), 2 μL human plasma and 2 μL inhibitor/DMSO/H2O were mixed lightly in an EP tube, then preincubated for 5 min at 37 °C in a thermostatic metal bath, and then the probe GP-BAN (2 μL, 10 mM) was added to start the reaction. After incubation for 30 min, the reaction was terminated by adding 200 μL of iced acetonitrile with vigorous shaking. Proteins were removed by centrifugation at 20,000 g for 10 min at 4 °C. 200 μL of supernatant was pipetted into a 96-well plate and the fluorescence signal of BAN (hydrolysis product of GP-BAN) was detected by multi-mode microplate reader (SpectraMax M4, Molecular Devices, Austria), setting the excitation wavelength at 430 nm, emission wavelength at 535 nm, and gain at 500. In addition, a blank control (enzyme was replaced with an equal volume of PBS solution) was also set up as background fluorescence; a negative control was replaced with an equal volume of 1 % (v/v) DMSO/ H2O solution as a 100 % control of enzyme activity. Three replicate wells were set up for each sample. The inhibition effect was expressed by the percentage of fluorescence intensity of the different inhibitor groups versus the control group.
IC50 determination of Inhibitors
The IC50 is the concentration of the inhibitor that reduces the enzyme activity by 50% range of each compound against DPP-IV was determined based on the results of the primary screening of monomers, and then a range of concentrations of monomeric compounds was formulated for plotting their dose-inhibition curves. IC50 values of inhibitors were determined using GraphPad lens 7.0 software Non-linear regression was evaluated.
Inhibition kinetic analysis
The of the compound with strong inhibitory ability (Kaempferol) against DPP-IV was assayed.
Subsequently, different concentrations of substrate/inhibitor were used to determine the corresponding reaction rate, and the second slope plot of the Lineweaver-Burk diagram was used as a function of the inhibitor to calculate the corresponding inhibition constant (Ki) value. All kinetic data were fitted by the following kinetic equations (a-c), for competitive (a), non-competitive (b) and mixed inhibition (c):
V =( VmaxS)/ [Km (1+I/Ki)+S] (a)
V =( VmaxS)/ [(Km +S)×(1+I/Ki)] (b)
V=( VmaxS)/ [(Km +S)×(1+I/αKi)] (c)
where V is the hydrolytic velocity of the reaction, Vmax is the maximum velocity. S and I are substrate GP-BAN and inhibitor Kaempferol concentrations, respectively. Ki is the inhibition constant of the tested inhibitor against the target PL and hCES1A; Km is the Michaelis constant (the substrate concentration at half of the Vmax ).
Statistical analysis
All measurements were performed in triplicate and all datas were shown as mean ± SD. The IC50 and Ki values of the compounds with strong inhibitory effect were evaluated by nonlinear regression with graphpad prism 7.0 software (GraphPad Software, Inc., La Jolla, USA).
Molecular docking simulation
Molecular docking simulation was used to decipher the binding mechanism of compound 27 (kaempferol) and DPP-IV at the molecular level. First, download the crystal structure of DPP-IV (PDB ID: 4N8D) from the protein date bank(https://www.rcsb.org/). The potential allosteric sites for DPP-IV were predicted from CavityPlus(http://www.pkumdl.cn:8000/cavityplus/index.php). Molecular docking simulations were finally performed using AutoDock Vina (version 1.1.2) based on Lamarckian genetic algorithm [29]. Following standard operating procedures, water and extraneous heteroatoms were removed, polar hydrogens were added, and Kollman charges were added. The center of the grid box was set to (28.353, 51.868, 66.961), the size was set to 80 × 80 × 80 Å3 with the spacing of 0.375 Å3. n the subsequent analysis of the results, favorable binding sites for the lowest binding energy conformation were pointed out.
Results
Inhibition of DPP-IV by Chinese patent medicines and Chinese medicines
The inhibitory effects of the six hypoglycemic Chinese patent medicines on DPP-IV were initially evaluated, as shown in When the concentrations of the six hypoglycemic Chinese patent medicines were less than 100 g/mL, there was no significant inhibitory effect on DPP-IV. When the concentrations were increased to 1000 g/mL, there was a certain inhibition effect on DPP-IV, and the residual activities of DPP-IV were 70.41%, 70.36%, 78.10%, 71.75%, 81.82%, and 82.74%, respectively. Further evaluation of the inhibition of DPP-IV by 33 Chinese medicines (removing duplicates) from six Chinese patent medicines revealed that most of these Chinese medicines showed no significant inhibition of DPP-IV at 1000 μg/mL, While Coptis chinensis and Cortex Moutanexhibited strong DPP-IV inhibition (residual activity <40%), the residual activity of DPP-IV was 27.90% and 35.44%, respectively. Therefore, the inhibition of DPP-IV by the monomeric components of Coptis chinensisand Cortex Moutancan be further evaluated, and in turn, natural DPP-IV inhibitors may be identified.
Evaluation of the inhibitory effects of 28 monomeric components on DPP-IV
Next, 16 monomeric components (1-16) of Coptis chinensis and 12 monomeric components [17-28] of Cortex Moutan were collected (Figure 1), and the inhibitory effects of these compounds on DPP-IV were evaluated. As shown in Figure 2, tetrandrine (4), magnolialine (8), chlorogenic acid (10), ferulic acid (11), protocatechuic acid (12), 3-phenylpropionic acid (14), vanillic acid (15), paeonol (17), paeonol glucoside (18), paeoniflorin (19), benzoyl paeoniflorin (20), paeoniflorin C (21), β-Sitosterol (24), catechin (28) in higher concentration (500 μM) showed no obvious inhibitory effect on DPP-IV. Berberine (1), coptisine (3), protocatechualdehyde (13), gentisic acid (16), gallic acid (22), oleanolic acid (23), betulinic acid (25) showed moderate inhibitory ability to DPP-IV, the residual activity of DPP-IV is between 50 and 80%. While palmatine (2), jatrorrhizine (5), sanguinarine (6), sanguinarine (7), wogonin (9), quercetin (26) and kaempferol (27) at 500 μM, the inhibitory effect on DPP-IV is strong, and the residual activity is less than 40%, and its half-inhibitory concentration (IC50) range can be further determined.
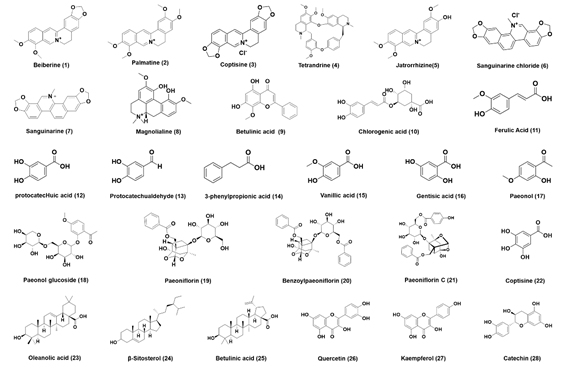
Figure 1. The chemical structures of monomer compounds in Coptis (1-16) and Cortex Moutan (17-28)
Inhibition of seven monomers on DPP-IV-mediated GP-BAN hydrolysis
Next, The IC50 of 5 kinds of Coptis chinensis monomers (wogonin, sanguinarine, sanguinarine, jatrorrhizine and palmatine), 2 kinds of Cortex Moutan monomer (kaempferol, quercetin) against DPP-IV was further determined, as shown in Table 2. The dose-dependent inhibition curve of DPP-IV-mediated GP-BAN response of Coptis chinensis monomers were shown in Figure 3. Four alkaloids, palmatine (2, IC50=264.80 μM), jatrorrhizine (5, IC50=227.00 μM), sanguinarine chloride (6, IC50=138.40 μM), sanguinarine (7, IC50=158.70 μM), and flavonoid, wogonin (9, IC50=62.47 μM), had a certain inhibitory effect against DPP-IV, this indicates that the hypoglycemic effect of Coptis chinensis is mainly alkaloids. In contrast, two flavonoids, quercetin (26, IC50=97.27 μM), kaempferol (27, IC50= 9.16 μM) had stronger inhibitory effects on DPP-IV, and the dose-dependent inhibition curves of DPP-IV-mediated GP-BAN response were shown in Figure 4. This encouraged us to identify the mechanism of inhibitory action of the strongest inhibitor, kaempferol (compound 27).
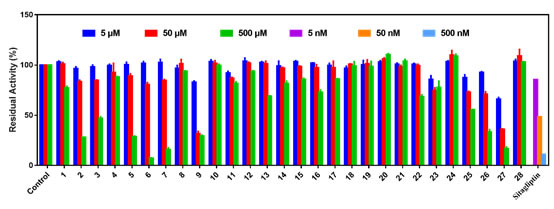
Figure 2. The inhibitory effects of 28 monomeric components on DPP-IV, Sitagliptin is a positive inhibitor of DPP-IV, and the test concentrations are 5 nM, 50 nM and 500 nM, respectively. Data are expressed as mean ± SD (n=3).
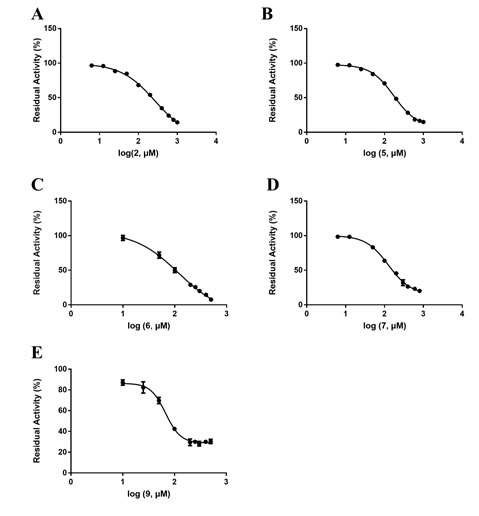
Figure 3. Dose-dependent inhibition curve of 5 monomers of Coptis chinensis against DPP-IV-mediated GP-BAN hydrolysis. Data are expressed as mean ± SD (n=3).
Table 1. The inhibitory effects of 6 kinds of Jiangtang granules and Chinese medicines on DPP-IV.
Chinese patent medicine(Chinese medicine) |
Residual activity /% |
Chinese patent medicine(Chinese medicine) |
Residual activity /% |
10 μg/mL |
100
μg/mL |
1000
μg/mL |
10 μg/mL |
100
μg/mL |
1000
μg/mL |
Liuwei Dihuang pill |
93.47 |
91.96 |
70.41 |
Yuquan Pill |
92.54 |
90.61 |
79.73 |
Jiangtangjia Tablet |
94.93 |
90.93 |
70.36 |
Jiangtangning Capsule |
100.15 |
94.39 |
81.82 |
Ganlu Xiaoke Capsule |
92.84 |
84.38 |
71.75 |
Xiaokeling tablet |
97.30 |
96.27 |
80.58 |
Liu
wei
Di
Huang
pill |
Cortex Moutan |
80.76 |
52.46 |
35.44 |
Yu
quan
Pill |
Trichosanthis Radix |
97.92 |
98.49 |
95.27 |
Poria Cocos |
99.90 |
100.15 |
97.84 |
Ophiopogon Japonicus |
99.50 |
100.87 |
95.26 |
Wine Cornus |
96.85 |
96.20 |
81.13 |
Radix Glycyrrhizae |
101.03 |
97.37 |
90.46 |
Rhizoma Dioscoreae |
88.94 |
91.14 |
89.87 |
Fructus Schisandrae Chinensis |
100.36 |
98.97 |
87.29 |
Radix Rehmanniae Preparata |
93.96 |
91.46 |
89.64 |
Puerarialobata |
94.86 |
93.68 |
84.58 |
Alisma Orientalis |
97.93 |
99.52 |
95.28 |
Rehmannia Glutinosa |
104.21 |
102.19 |
85.92 |
Jiang
Tang
jia
Tablet |
Rhizoma Polygonati |
106.12 |
96.47 |
95.56 |
Jiang
Tang
ning Capsule |
Radix Glycyrrhizae |
101.03 |
97.37 |
90.46 |
Rehmannia Glutinosa |
104.21 |
102.19 |
85.92 |
Fructus Corni |
104.40 |
96.15 |
66.29 |
Radix Pseudostellariae |
96.18 |
94.23 |
95.80 |
Stigma Maydis |
84.90 |
82.80 |
63.90 |
Trichosanthis Radix |
97.92 |
98.49 |
95.27 |
Rehmannia Glutinosa |
104.21 |
102.19 |
85.92 |
Astragalus Membranaceus |
93.04 |
97.52 |
95.49 |
Cortex Lycii |
101.76 |
98.45 |
81.56 |
Ganlu Xiaoke Capsule |
Radix Rehmanniae Preparata |
93.96 |
91.46 |
89.64 |
Ophiopogon Japonicus |
99.50 |
100.87 |
95.26 |
Rehmannia Glutinosa |
104.21 |
102.19 |
85.92 |
Poria Cocos |
99.90 |
100.15 |
97.84 |
Lycii Fructus |
100.33 |
97.90 |
99.22 |
Trichosanthis Radix |
97.92 |
98.49 |
95.27 |
Cortex Lycii |
101.76 |
98.45 |
81.56 |
Astragalus Membranaceus |
93.04 |
97.52 |
95.49 |
Fructus Corni |
104.40 |
96.15 |
66.29 |
Rhizoma Anemarrhenae |
94.97 |
88.57 |
89.81 |
Radix Scrophulariae |
104.80 |
98.08 |
96.41 |
Linn Gypsum |
93.52 |
95.79 |
94.31 |
Ginseng Radix et Rhizoma |
91.40 |
91.88 |
91.52 |
Rhizoma Dioscoreae |
88.94 |
91.14 |
89.87 |
Radix Codonopsis |
97.90 |
88.48 |
79.44 |
Ginseng Radix et Rhizoma |
91.40 |
91.88 |
91.52 |
Astragalus Membranaceus |
93.04 |
97.52 |
95.49 |
Xiao
Ke
ling
tablet |
Ophiopogon Japonicus |
99.50 |
100.87 |
95.26 |
Semen Cuscutae |
89.24 |
79.92 |
52.99 |
Fructus Schisandrae Chinensis |
100.36 |
98.97 |
87.29 |
Trichosanthis Radix |
97.92 |
98.49 |
95.27 |
Cortex Moutan |
80.76 |
52.46 |
35.44 |
Radix Angelicae Sinensis |
95.08 |
94.88 |
89.46 |
Rhizoma Coptidis |
99.54 |
82.99 |
27.90 |
Rhizoma Coptidis |
99.54 |
82.99 |
27.90 |
Astragalus Membranaceus |
93.04 |
97.52 |
95.49 |
Rhizoma Atractylodis Macrocephalae |
97.27 |
99.98 |
94.79 |
Radix Ginseng Rubra |
105.68 |
100.99 |
108.88 |
Ootheca Mantidis |
102.54 |
97.81 |
89.97 |
Poria Cocos |
99.90 |
100.15 |
97.84 |
Radix Asparagi |
102.86 |
98.42 |
99.39 |
Rehmannia Glutinosa |
104.21 |
102.19 |
85.92 |
Ophiopogon Japonicus |
99.50 |
100.87 |
95.26 |
Linn Gypsum |
93.52 |
95.79 |
94.31 |
Alisma Orientalis |
97.93 |
99.52 |
95.28 |
Lycii Fructus |
100.33 |
97.90 |
99.22 |
Poria Cocos |
99.90 |
100.15 |
97.84 |
Trichosanthis Radix |
97.92 |
98.49 |
95.27 |
*sitagliptin |
85.43 |
48.49 |
11.25 |
*Sitagliptin: a positive inhibitor of DPP-IV and was tested at concentrations of 2 ng/mL, 20 ng/mL, 200 ng/mL.
Table 2. Evaluation of the inhibitory effect of 7 monomers in Coptis and Moutan cortex on DPP-IV.
The concentration range of compound 2, 5, 6, 7 was 0-1000 μM, the concentration range of compound 9, 26, 27 was 0-500 μM.
|
Compound |
MS |
Enzyme source |
Target enzyme |
IC50 (μM) |
2 |
Palmatine |
352.40 |
Human Plasma |
DPP-IV |
264.80 ± 11.89 |
5 |
Jjatrorrhizine |
465.28 |
Human Plasma |
DPP-IV |
227.00 ± 18.13 |
6 |
Sanguinarine chloride |
367.78 |
Human Plasma |
DPP-IV |
138.40 ± 12.82 |
7 |
Sanguinarine |
102.55 |
Human Plasma |
DPP-IV |
158.70 ± 15.10 |
9 |
Wogonin |
284.26 |
Human Plasma |
DPP-IV |
62.47 ± 11.12 |
26 |
Quercetin |
302.24 |
Human Plasma |
DPP-IV |
97.27 ±10.71 |
27 |
Kaempferol |
286.24 |
Human Plasma |
DPP-IV |
9.16 ± 0.69 |
The inhibitory kinetics of kaempferol on DPP-IV-mediated hydrolysis of GP-BAN
In order to explore the inhibitory mechanism of the strongest inhibitor (compound 27) against DPP-IV, a set of inhibition kinetics experiments were carried out with different concentrations of inhibitor (compound 27) and substrate (GP-BAN). As shown in Figure 5, inhibition kinetic assays clearly show that compound 27 inhibit the DPP-IV-mediated hydrolysis of GP-BAN in plasma in a non-competitive manner, and the inhibition constants (Ki) was 11.68 μM.
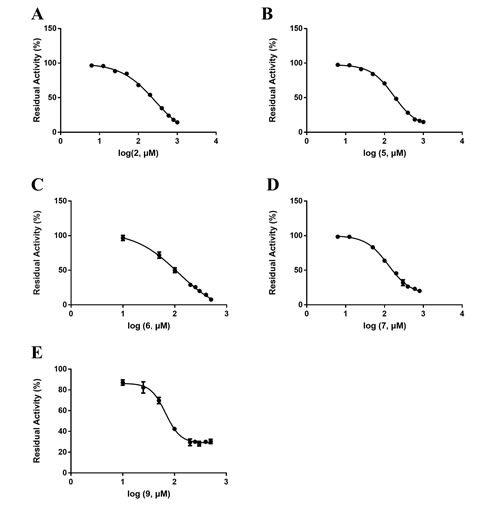
Figure 4. Dose-dependent inhibition curve of 2 flavonoids of Cortex Moutan against DPP-IV-mediated GP-BAN hydrolysis. Data are expressed as mean ± SD (n=3).
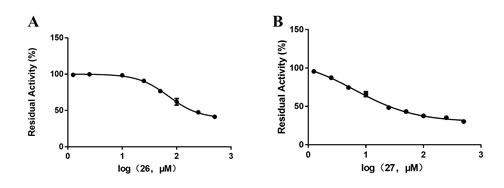
Figure 5. A) The Lineweave-Burk plots of compound 27 against DPP-IV-catalyzed GP-BAN hydrolysis. B) The second plot of slopes from A.(R2=0.9963) Data are expressed as mean ± SD (n=3).
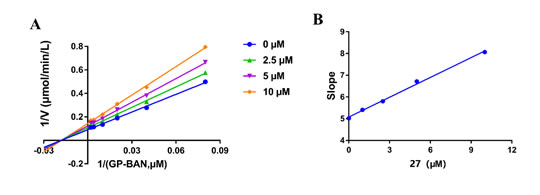
Figure 6. A). Stereoscopic view(left) and detailed view(right) of kaempferol docking with DPP-IV. B) 2D view of kaempferol docking with DPP-IV.
Docking simulation
In order to deeply explore the interaction mode between the inhibitor compound 27 and the target DPP-IV, molecular docking simulation was carried out with the allosteric site of DPP-IV. The conformation of the ligand with the lowest docking binding energy with DPP-IV was analyzed, and the binding energy was -7.2 kcal/mol. The detailed interaction was shown in Figure 6, and the OH connected at position 3 on the mother nucleus of flavonoids can form hydrogen bond with Ser720 at a distance of 1.5 Å, and the benzene ring on the mother nucleus can also form π - π interaction with Phe730. In addition, the benzene ring connected at position 2 can form π - π interaction with Phe713. In addition, it can also form van der Waals force with amino acids such as His704, Ser716, Ala717 And Val724. These interactions indicate that compound 27 inhibits the activity of the enzyme by binding to amino acids other than the DPP-IV active center.
Discussion
At present, domestic scholars have made many achievements in the modernization of traditional Chinese medicine, but the characteristics and complexity of traditional Chinese medicine with multi-component, multi-target and multi-channel action still exist [30]. Coptis chinensis was first published in Divine Farmer’s Materia Medica (Shennong Bencao Jing) Shennong’s Classic of Materia Medica in China, and was listed as top grade [31], with a wide range of pharmacological activities, including antibacterial, antiviral, antiatherosclerotic, antihypertensive, antiinflammatory, antitumor, antidiabetic, anti-hepatic steatosis, etc [32-34]. Studies have shown that Coptis chinensis has a variety of active components, in addition to alkaloids as the main components, it also contains organic acids, lignans, flavonoids, volatile oils, etc [35, 36]. Studies have shown that berberine in Coptis chinensis can increase the activity of protein kinase C (PKC) to activate the expression of insulin receptor mRNA, promote insulin secretion and lower blood sugar [37]; in addition, berberine can enhance the glucose transporter The expression of 1 (glucose transporter 1, GLUT1) promotes the uptake of glucose by adipocytes and achieves hypoglycemic effect [38]. Cortex Moutan was recorded in several famous Chinese medical books, like Compendium of Materia Medica (Ben Cao Gang Mu),Shen Nong's Herbal Classic (Shen Nong Ben Cao Jing), etc. and its pharmacological effects include anti-inflammatory, antioxidant, anti-tumor, and anti-diabetic. The main components contained are terpenes, phenols, glycosides, tannins, flavonoids, etc., such as paeoniflorin, oxidized paeoniflorin, gallic acid, paeonol, etc. [39]. The study found that Cortex Moutan can inhibit the activity of advanced glycation end products (AGEs) in vitro, and the five components of trigalloyl-glucose, tetragalloyl-glucose, galloylpaeoniflorin, hexagalloyl-glucose, and benzoylpaeoniflorin may be the active substance of Cortex Moutan in the prevention and treatment of diabetic nephropathy [40]. Furthermore, a novel heteropolysaccharide (MC-pa) has protective effects against diabetic nephropathy (DN) [41]. Although Coptis chinensis and Cortex Moutan have pharmacological effects of lowering blood sugar, there are few studies on whether they lower blood sugar based on DPP-IV.
Based on the DPP-IV target, this study found that Coptis chinensis and Cortex Moutan have strong inhibitory effects on DPP-IV through the self-developed high-efficiency screening method of DPP-IV inhibitors, and their inhibitory material basis mainly includes alkaloids (palmatine, jatrorrhizine, sanguinarine and sanguinarine) and flavonoid (wogonin, kaempferol and quercetin) compounds. Among them, kaempferol has the strongest inhibitory effect on DPP-IV with IC50 of 9.26 μM and Ki of 11.68 μM. Further inhibition kinetics and molecular docking studies revealed that kaempferol is a potent non-competitive DPP-IV inhibitor.
Now there are mainly some synthetic drugs on the market, including all the listed drugs, and there are many reports based on the structure-activity relationship of synthetic drugs, such as pyrrolidine, aminopiperidine, xanthine, carbonyl, cyanobenzene and other groups are crucial for inhibiting the activity of DPP-IV important [42,43]. A large number of molecular docking shows aromatic nucleus (such as pyrimidine, benzimidazole, quinoline, isoquinoline, etc.) can carry out π-π interaction with Arg125. In addition, the xanthine nucleus forms hydrogen bonds with Ser630 and forms π-π interactions with Tyr547. Benzene, cyanobenzene, butyl or other small cyclic substituents occupy the S1 pocket and can hydrophobic interact with Ser630/His740. In addition, aminopiperidine or piperazine are essential for DPP-IV inhibitory activity, which can form a salt bridge with Glu205, Glu206 or Tyr662 in the S2 pocket [42,44,45]. Most of the inhibitors reported above are competitive inhibitors [24,27], while kaempferol is a non-competitive inhibitor, which mainly binds to amino acids (Ser720, His704, Ser716, etc.) outside the active center of DPP-IV to inhibit the activity of the enzyme.
Conclusion
In summary, this study started with DPP-IV, an important target of type II diabetes, and found the active ingredients that inhibit DPP-IV from Coptis chinensis and Cortex Moutan. 5 kinds of monomer components of Coptis chinensis (wogonin, sanguinarine, sanguinarine, jatrorrhizine and palmatine), 2 kinds of monomer components of Cortex Moutan (kaempferol, quercetin), which have positive effects on DPP-IV. Strong inhibitory effect, among which kaempferol has the strongest inhibitory effect on DPP-IV, with IC50 of 9.26 μM and Ki of 11.68 μM. Further studies on inhibition kinetics and molecular docking showed that kaempferol is an effective non-competitive inhibitor of DPP-IV, and the above studies can provide reference for the study of its hypoglycemic mechanism.
Financial support
Authors are grateful to NSF of China (21602219), the National Science and Technology Major Project of China (2018ZX09731016), the National Key Research and Development Program of China (2017YFC1702000), and the Special Project of Postgraduate Innovation Training of Shanghai University of Traditional Chinese Medicine (Y2020068).
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author Contributions
Jing Zhang and Ci-Qin Li, performed most of the experiments. Li-Juan Tian, prepared the test sample reagents, materials. Xing-Kai Qian, constructed the screening method. An-Qi Wang, performed the Molecular Docking. Cong-Cong Zhang and Li-Wei Zou, designed and analyzed the experiments. Li-Wei Zou, Jing Zhang, Ci-Qin Li and Shi-Yang Li, prepared the manuscript with contributions from all authors.
Abbreviations
AGEs: Advanced Glycation End Products
Ala: Alanine
BAN: N-butyl-4-amino-1,8-naphthalimide
DN: Diabetic Nephropathy
DPP-IV: Dipeptidyl peptidase-IV
GLP-1: Glucagon-like peptide-1
GLUT1: Glucose transporter 1
GP-BAN: Gly-Pro-N-butyl-4-amino-1,8-naphthalimide
HTS: High-Throughput Screening
PKC: Protein Kinase C
Pro: Proline
T2DM: Type 2 Diabetes Mellitus
References
- Karaa A, Goldstein A (2015) The spectrum of clinical presentation, diagnosis, and management of mitochondrial forms of diabetes. Pediatr Diabetes 16: 1-9. [Crossref]
- Nongonierma AB, FitzGerald RJ (2019) Features of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from dietary proteins. J Food Biochem 43: e12451.
- Ceriello A, Inagaki N (2017) Pharmacokinetic and pharmacodynamic evaluation of linagliptin for the treatment of type 2 diabetes mellitus, with consideration of Asian patient populations. J Diabetes Investig 8: 19-28. [Crossref]
- Sun ZG, Li ZN, Zhu HL (2020) The Research Progress of DPP-4 Inhibitors. Mini Rev Med Chem 20: 1709-1718. [Crossref]
- Scheen AJ (2015) A review of gliptins for 2014. Expert Opin Pharmacother 16: 43-62. [Crossref]
- Chen SJ, Jiaang WT (2011) Current advances and therapeutic potential of agents targeting dipeptidyl peptidases-IV, -II, 8/9 and fibroblast activation protein. Curr Top Med Chem 11: 1447-1463.
- Kwok AJ, Mashar M, Khavandi K, Sabir I (2014) DPP-IV inhibitors: Beyond glycaemic control? Trends Cardiovasc Med 24: 157-164. [Crossref]
- Wang DD, Qian X, Li H, Jia G, Jin Q, et al. (2021) Sensing and imaging of exosomal CD26 secreted from cancer cells and 3D colorectal tumor model using a novel near-infrared fluorogenic probe. Mater Sci Eng C Mater Biol Appl 130: 112472. [Crossref]
- Soni R, Soman SS (2018) Design and synthesis of aminocoumarin derivatives as DPP-IV inhibitors and anticancer agents. Bioorg Chem 79: 277-284. [Crossref]
- Juillerat-Jeanneret L (2014) Dipeptidyl peptidase IV and its inhibitors: therapeutics for type 2 diabetes and what else? J Med Chem 57: 2197-2212.
- Hinke SA, Gelling RW, Pederson RA, Manhart S, Nian C, et al. (2002) Dipeptidyl peptidase IV-resistant [D-Ala(2)]glucose-dependent insulinotropic polypeptide (GIP) improves glucose tolerance in normal and obese diabetic rats. Diabetes 51: 652-661. [Crossref]
- Meier JJ (2012) GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 8: 728-742. [Crossref]
- Tsai TY, Hsu T, Chen C, Cheng J, Yeh T, et al. (2009) Novel trans-2-aryl-cyclopropylamine analogues as potent and selective dipeptidyl peptidase IV inhibitors. Bioorg Med Chem 17: 388-399.
- Biftu T, Scapin G, Singh S, Feng D, Becker JW, et al. (2007) Rational design of a novel, potent, and orally bioavailable cyclohexylamine DPP-4 inhibitor by application of molecular modeling and X-ray crystallography of sitagliptin. Bioorg Med Chem Lett 17: 3384-3387. [Crossref]
- Gong Q, Shi W, Li L, Wu X, Ma H, et al. (2016) Ultrasensitive Fluorescent Probes Reveal an Adverse Action of Dipeptide Peptidase IV and Fibroblast Activation Protein during Proliferation of Cancer Cells. Anal Chem 88: 8309-8314. [Crossref]
- Quek A, Kassim NK, Ismail A, Mohammad Latif MA, Shaari K, et al. (2020) Identification of Dipeptidyl Peptidase-4 and α-Amylase Inhibitors from Melicope glabra (Blume) T. G. Hartley (Rutaceae) Using Liquid Chromatography Tandem Mass Spectrometry, In Vitro and In Silico Methods. Molecules 26: 1-16. [Crossref]
- Yang M, Shen C, Zhu S, Zhang Y, Jiang H, et al. (2022) Chinese patent medicine Aidi injection for cancer care: An overview of systematic reviews and meta-analyses. J Ethnopharmacol 282: 114656. [Crossref]
- Zheng W, Wang G, Zhang Z, Wang Z, Ma K (2020) Research progress on classical traditional Chinese medicine formula Liuwei Dihuang pills in the treatment of type 2 diabetes. Biomed Pharmacother 121: 109564. [Crossref]
- He D, Huang J, Zhang Z, Du Q, Peng W, et al. (2019) A Network Pharmacology-Based Strategy For Predicting Active Ingredients And Potential Targets Of LiuWei DiHuang Pill In Treating Type 2 Diabetes Mellitus. Drug Des Devel Ther 13: 3989-4005. [Crossref]
- Zhang K, Weng H, Yang J, Wu C, et al. (2020) Protective effect of Liuwei Dihuang Pill on cisplatin-induced reproductive toxicity and genotoxicity in male mice. J Ethnopharmacol 247: 112269. [Crossref]
- Qi LW, Liu E, Chu C, Peng Y, Cai H, et al. (2010) Anti-diabetic agents from natural products--an update from 2004 to 2009. Curr Top Med Chem 10: 434-57. [Crossref]
- Peng S, Xie Z, Zhang X, Xie C, Kang J, et al. (2021) Efficacy and Safety of the Chinese Patent Medicine Yuquan Pill on Type 2 Diabetes Mellitus Patients: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med 2021: 2562590. [Crossref]
- Liu L, Zhang Y, Zhu Z, Yu Z, Bao P, et al. (2021) Yuquan pill enhance the effect of Western medicine in treatment diabetic nephropathy: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 100: e27555. [Crossref]
- Zhang J, Qian X, Song P, Li X, Wang A, et al. (2021) A high-throughput screening assay for dipeptidyl peptidase-IV inhibitors using human plasma. Anal Methods 13: 2671-2678.
- Jin Q, Song L, Ding L, Zhang J, Wang D, et al. (2022) High-throughput optical assays for sensing serine hydrolases in living systems and their applications. TrAC Trends in Analytical Chemistry 152: 116620.
- Zou LW, Wang P, Qian X, Feng L, Yang Y, et al. (2017) A highly specific ratiometric two-photon fluorescent probe to detect dipeptidyl peptidase IV in plasma and living systems. Biosens Bioelectron 90: 283-289.
- Qian XK, Zhang J, Li X, Song P, Zou L (2022) Research Progress on Dipeptidyl Peptidase Family: Structure, Function and Xenobiotic Metabolism. Curr Med Chem 29: 2167-2188. [Crossref]
- Ma H, Qian X, Zhang J, Jin Q, Zou L, et al. (2020) Accurate and sensitive detection of dipeptidyl peptidase-IV activity by liquid chromatography with fluorescence detection. Analytical Methods 12: 848-854.
- Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31: 455-461. [Crossref]
- Sun J, Han T, Yang T, Chen Y, Huang J (2021) Interpreting the Molecular Mechanisms of Yinchenhao Decoction on Hepatocellular Carcinoma through Absorbed Components Based on Network Pharmacology. Biomed Res Int 2021: 6616908. [Crossref]
- Wang J, Wang L, Lou G, Zeng H, Hu J, et al. (2019) Coptidis Rhizoma: a comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. Pharm Biol 57: 193-225. [Crossref]
- Meng FC, Wu Z, Yin Z, Lin L, Wang R, et al. (2018) Coptidis rhizoma and its main bioactive components: recent advances in chemical investigation, quality evaluation and pharmacological activity. Chin Med 13: 13. [Crossref]
- Wu J, Zhang H, Hu B, Yang L, Wang P, et al. (2016) Coptisine from Coptis chinensis inhibits production of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. Eur J Pharmacol 780: 106-114. [Crossref]
- Lv X, Li Y, Tang C, Zhang Y, Zhang J, et al. (2016) Integration of HPLC-based fingerprint and quantitative analyses for differentiating botanical species and geographical growing origins of Rhizoma coptidis. Pharm Biol 54: 3264-3271. [Crossref]
- Zhang Q, Piao XL, Piao XS, Lu T, Wang D, et al. (2011) Preventive effect of Coptis chinensis and berberine on intestinal injury in rats challenged with lipopolysaccharides. Food Chem Toxicol 49: 61-69. [Crossref]
- Liu Y, Wang B, Shu S, Li Z, Song C, et al. (2021) Analysis of the Coptis chinensis genome reveals the diversification of protoberberine-type alkaloids. Nat Commun 12: 3276. [Crossref]
- Kong WJ, Zhang H, Song D, Xue R, Zhao W, et al. (2009) Berberine reduces insulin resistance through protein kinase C-dependent up-regulation of insulin receptor expression. Metabolism 58: 109-119. [Crossref]
- Cok A, Plaisier C, Salie MJ, Oram DS, Chenge J, et al. (2011) Berberine acutely activates the glucose transport activity of GLUT1. Biochimie 93: 1187-1192. [Crossref]
- Wang Z, He C, Peng Y, Chen F, Xiao P, et al. (2017) Origins, Phytochemistry, Pharmacology, Analytical Methods and Safety of Cortex Moutan (Paeonia suffruticosa Andrew): A Systematic Review. Molecules 22: 946. [Crossref]
- Chen J, Zhang L, Zhang MH, Zhao D, Yuan JR, et al. (2016) Inhibitory effects of extracts from Moutan Cortex on formation of advanced glycation end-products (AGEs) in vitro and specificity screening of its potential active components. Zhongguo Zhong Yao Za Zhi 41: 891-897. [Crossref]
- Lian Y, Zhu M, Chen J, Yang B, Lv Q, et al. (2021) Characterization of a novel polysaccharide from Moutan Cortex and its ameliorative effect on AGEs-induced diabetic nephropathy. Int J Biol Macromol 176: 589-600. [Crossref]
- Costante R, Stefanucci A, Carradori S, Novellino E, Mollica A, et al. (2015) DPP-4 inhibitors: a patent review (2012 - 2014). Expert Opin Ther Pat 25: 209-236. [Crossref]
- Patel BD, Bhadada SV, Ghate MD (2017) Design, synthesis and anti-diabetic activity of triazolotriazine derivatives as dipeptidyl peptidase-4 (DPP-4) inhibitors. Bioorg Chem 72: 345-358. [Crossref]
- Hussain H, Abbas G, Green IR, Ali I (2019) Dipeptidyl peptidase IV inhibitors as a potential target for diabetes: patent review (2015-2018). Expert Opin Ther Pat 29: 535-553. [Crossref]
- Singh AK, Patel PK, Choudhary K, Joshi J, Yadav D, et al. (2020) Quercetin and Coumarin Inhibit Dipeptidyl Peptidase-IV and Exhibits Antioxidant Properties: In Silico, In Vitro, Ex Vivo. Biomolecules 10: 207. [Crossref]






