Chimeric antigen receptor (CAR) T cell therapy is a promising new immunotherapy that reprograms patient T lymphocytes to specifically recognize and kill tumor cells. CAR T cell therapy has produced some dramatic responses in acute lymphoblastic leukemia and lymphomas, but responses have been less spectacular in solid tumors. To make CAR T cell therapy effective for solid tumors, CAR T cells must overcome an immune-suppressive tumor microenvironment (TME) that attenuates CAR T cell function. This review provides insights into mechanisms of CAR T cell therapy resistance with respect to the TME and offers strategies for improving CAR T cell therapy by targeting immune-suppressive factors in tumors.
cancer, CAR T cells, tumor microenvironment
CAR T cell therapy is a promising new immunotherapy that provides potential curative treatments for cancer. CARs consist of a tumor-targeting monoclonal antibody-derived single chain variable fragment (scFv) fused to a T cell receptor-derived cytoplasmic signaling domain CD3ζ and one or more domains derived from co-stimulatory T cell receptors CD28, 4-1BB, or OX40 (Figure 1A) [1]. When expressed by T cells, CARs redirect T cell specificity to antigens expressed on cancer cells. To deliver CAR T cell therapy, patient T cells are genetically modified to express CAR and then amplified ex vivo to numbers suitable for adoptive cell therapy [2]. Recent clinical data provided strong evidence that T cells from patients with B cell malignancies can successfully be redirected to initiate an effective anti-tumor response even at advanced stages of the disease [3-15]. In relapsed or refractory B cell acute lymphoblastic leukemia (B-ALL) and certain types of lymphoma, this strategy has led to dramatic complete responses in more than 80% of patients treated with CD19-targeting (CAR19) T cell therapies [3-15]. The FDA has recently approved the CAR T cell products axicabtagene ciloleucel and tisagenlecleucel to treat relapsed or refractory B-ALL and diffuse large B cell lymphoma (DLBCL) [8,12,16,17]. CAR T cells can thus be considered as “designer drugs” that are personalized to patients’ needs and manufactured to clinical standards.
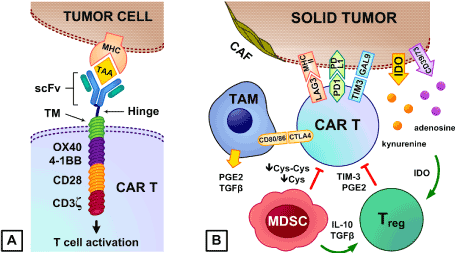
Figure 1A. Chimeric antigen receptor (CAR) T cells. Derived from tumor-specific monoclonal antibodies, the extracellular single chain variable fragment (scFv) recognizes tumor-associated antigens (TAA) and is connected via the hinge to the transmembrane domain (TM) that anchors to the CAR T cell’s plasma membrane. Attached to the TM are intracellular co-stimulatory (CD28, 4-1BB, or OX40) and T cell receptor CD3ζ-derived signaling domains that activate the CAR T cell
Figure 1B. Immune-suppressive cells in the TME lower CAR T anti-tumor activity. Regulatory T cells (Tregs) inhibit CAR T cell proliferation and cytokine production via TIM-3 and prostaglandin E2 (PGE2). Myeloid-derived suppressor cells (MDSCs) suppress T cell activation via cystine (Cys-Cys) and cysteine (Cys) deprivation [72], and facilitate Tregs recruitment and expansion via IL-10 and TGF-β [73]. Type II tumor-associated macrophages (TAMs) release PGE2 and TGF-β, and express CD80/CD86 that preferentially binds to the inhibitory CTLA-4 receptor in CAR T cells. Cancer-associated fibroblasts (CAFs) physically prevent CAR T cells from accessing tumor antigens. Tumor cells express MHC class II, galectin-9 (Gal9), and PD-L1 that bind to the LAG3, TIM-3, and PD-1 receptors on CAR T cells to promote CAR T cell exhaustion and apoptosis [71]. Immune-suppressive metabolites kynurenine and adenosine are produced via indolamine-2,3-dioxygenase (IDO) and CD39/CD73, and IDO is involved in activation of Tregs [74]
Using viral vectors to transfer CAR genes to T cells requires complex protocols that are time-consuming and expensive. Compared to viral vectors, non-viral transposon-based gene delivery systems offer a simpler and cheaper alternative for CAR T manufacture with no infectious potential [18-20]. Novel CAR T cells generated using Sleeping Beauty (SB) and PiggyBac (PB) transposon/transposase systems have demonstrated strong efficacy against leukemia cells in preclinical mouse models [18-21]. This preclinical data provided the basis for testing transposon-based CAR T cells in clinical trials in USA, Australia and China [21-23]. Importantly, the decreased cost and complexity of non-viral genome modification methods can widen patient access to CAR T cell therapies by increasing the number of hospitals capable of implementing them.
Recent clinical studies have shown that CAR T cells can cure select patients with cancer, while others experience transient or no clinical benefit [4,5,10,15,24,25]. Short duration of remission in patients treated with CAR T cells can be associated with functional CAR T cell exhaustion in the immune-suppressive TME [6,7,26,27]. The immune-suppressive TME considerably reduces the efficacy of CAR T cell therapy against solid tumors such as prostate [28-30], ovarian [31-35], breast [36-39], pancreatic [40-44], and brain [45] cancers. Disialoganglioside (GD2)-targeting CARs incorporating CD28 and OX40 co-stimulatory domains showed efficient CAR T cell infiltration of neuroblastoma tumors; however, the suppression of tumor growth was marginal, suggesting that CAR T cell function is compromised by the immune-suppressive TME [46,47].
The immune-suppressive TME is enriched with regulatory immune cells such as regulatory T cells (Tregs) [48-55], myeloid-derived suppressor cells (MDSCs) [36,39,56,57], tumor-associated macrophages (TAMs) [58-60], and cancer-associated fibroblasts (CAFs) [35,61]. These regulatory immune cells inhibit CAR T cells by releasing suppressive factors such as TGF-β [28,30,58,62], IL-4 [43,63,64], IL-10 [40], prostaglandin E2 (PGE2) [65], and immune-suppressive metabolites such as kynurenine and adenosine via indolamine-2,3-dioxygenase (IDO) [59,66,67] and CD39/CD73 respectively [38,68-70]. Additionally, tumor cells often down-regulate T cell co-stimulatory ligands that would normally promote CAR T cell function, while up-regulating immune-suppressive ligands (MHC class II, galectin-9, PD-L1 and CD86) that activate immune checkpoints (LAG3, TIM-3, PD-1 and CTLA-4) in adoptively transferred CAR T cells (Figure 1B) [71]. Chemoresistant and chemorefractory pediatric B-ALLs exhibit significant interpatient heterogeneity in the expression of 35 genes that encode T cell co-stimulatory and inhibitory ligands, and in vitro models showed association of CD86, CD70, ICOSL, OX40, and IL-10 with CAR T cell expansion and exhaustion [26]. B-ALLs exhibit low expression of PD-L1 and CD80/CD86, which activates the PD-1 and CTLA-4 checkpoints in CAR T cells [26]. Unlike B-ALLs, T cell acute lymphoblastic leukemia (T-ALL) cells express high levels of PD-L1 and often CD80/CD86, and so can be considered as more immune-suppressive than B-ALL in this respect [26]. B-ALLs from some patients, however, express MHC class II and galectin-9 (Gal9) that bind to the LAG3 and TIM-3 receptors on CAR T cells to promote CAR T cell exhaustion and apoptosis [71].
CARs with multiple co-stimulatory domains and/or genome-edited checkpoint receptors have been engineered to mitigate the immune-suppressive TME [70,75-83]. New approaches also combine CAR T cell therapy with chemotherapeutic drugs, epigenetic modulators, or targeted drugs that attenuate immune suppression in the TME in addition to direct anti-tumor activity [32,36,45,47,48,50,67,84-89]. Some epigenetic drugs up-regulate the tumor’s antigen expression for targeting by CAR T cells, up-regulate T cell co-stimulatory ligands, or induce type I interferon responses in tumors against pro-viruses integrated into target cell genomes [90]. The hypomethylating agent 5-azacitidine (AZA) sensitizes leukemia and lymphoma cells to CAR T cell therapy by modulating the TME in leukemia and inducing OX40L to promote CAR T cell function [20,86]. Other epigenetic modulators down-regulate the immune-suppressive ligands that activate specific immune checkpoints in CAR T cells. JQ1, a potent small-molecule bromodomain and extra terminal domain (BET) inhibitor: down-regulates PD-L1 expression in neuroblastoma and sensitizes neuroblastoma cells to CAR T cell therapy [47,85]. JQ1 also promotes CAR T cell activity by up-regulating interferon regulatory factor 7 (IRF7) signalling to activate type I interferon responses [47,85].
Combining CAR T cells with specific targeted drugs have been shown to promote CAR T cell function [67,84,87,89]. Ibrutinib is a small-molecule drug that binds permanently to Bruton’s tyrosine kinase and is used to treat chronic lymphocytic leukemia (CLL). CLL patients showed prolonged remission after combined treatment with ibrutinib and CAR19 T cells [84,87,89]. Ibrutinib induced mobilization of the disease into blood or bone marrow, where it is highly responsive to CAR T therapy [84,87,89]. Lenalidomide, another targeted drug for treating multiple myeloma (MM), delayed the onset of CAR T cell functional exhaustion in the immune-suppressive TME by potentiating CAR T cells that target B cell maturation antigens in MM [67].
Another promising strategy involves using checkpoint inhibitors to rejuvenate exhausted CAR T cells [70,75-83]. Up-regulation of specific checkpoint ligands such as PD-L1 in inflamed TME induces premature CAR T cell exhaustion. PD-1 checkpoint blockade with PD-1 and/or PD-L1 antagonistic antibodies [91-94] acts to rescue CAR T cells from exhaustion and improve their cytolytic activity in melanoma [77,95-97]. PD-1 checkpoint inhibitors induced remission in B-ALL patients that relapsed following CAR T cell therapy [98], but did not promote CAR T cell efficacy in recent neuroblastoma clinical studies [99], suggesting that additional factors in the TME may be involved in CAR T cell exhaustion in neuroblastoma patients.
Agents targeting Tregs, MDSCs, TAMs, and CAFs in the TME are currently being investigated in the context of CAR T cell therapy against hematological and solid tumors [100-107]. Macrophage colony stimulating factor 1 (CSF1) and granulocyte macrophage colony stimulating factor (GM-CSF) antagonists were shown to inhibit TAMs and MDSCs and promote CAR T cell function [100-102].
CAR T cell therapy has been a major breakthrough in cancer treatment. Despite encouraging clinical results in certain hematological malignancies, high resistance to CAR T cell therapies has often been reported in patients with solid tumors. Multiple mechanisms contributing to CAR T resistance have led to the design of complex therapeutic strategies to avoid immune suppression in the TME of solid tumors and to increase tumor cell susceptibility to CAR T cell attack. Resistance mechanisms need to be examined in different contexts in order to design effective therapeutic combinations and improve the efficacy of CAR T cell therapy.
This work was funded by Kids Cancer Project grants. The authors thank Dr Kenneth Micklethwaite from Westmead Institute of Medical Research, Sydney, for providing constructive discussion of the manuscript.
All authors participated in the preparation, writing and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
- Sadelain M, Brentjens R, Rivière I (2013) The basic principles of chimeric antigen receptor design. Cancer discovery 3: 388-398. [Crossref]
- Lee DW, Barrett DM, Mackall C, Orentas R, Grupp SA (2012) The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer. Clin Cancer Res 18: 2780-2790.
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X (2013) CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 5: 177ra38.
- Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K (2014) Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6: 224ra25.
- Gardner RA, Finney O, Annesley C, Brakke H, Summers C (2017) Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 129: 3322-3331.
- Kochenderfer JN, Somerville RPT, Lu T, Shi V, Bot A (2017) Lymphoma remissions caused by Anti-CD19 chimeric antigen receptor T cells are associated with high serum Interleukin-15 Levels. J Clin Oncol 35: 1803-1813.
- Kochenderfer JN, Somerville RPT, Lu T, Yang JC, Sherry RM (2017) Long-Duration complete remissions of diffuse large b cell lymphoma after Anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther 2017;25(10):2245-2253.
- Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ (2019) Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 20: 31-42.
- Locke FL, Neelapu SS, Bartlett NL, Siddiqi T, Chavez JC (2017) Phase 1 Results of ZUMA-1: A Multicenter Study of KTE-C19 Anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther 25: 285-295.
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371: 1507-1517.
- Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M (2018) Tisagenlecleucel in children and young adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 378: 439-448.
- Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ (2017) Axicabtagene Ciloleucel CAR T-Cell Therapy in refractory large B-Cell Lymphoma. N Engl J Med 377: 2531-2544. [Crossref]
- Park JH, Rivière I, Gonen M, Wang X, Sénéchal B (2019) Long-Term follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. The New England journal of medicine 378: 449-459.
- Turtle CJ, Hanafi LA, Berger C, Gooley TA (2016) CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 126: 2123-2138.
- Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B (2016) Immunotherapy of non-Hodgkin's lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 8: 355ra116.
- FDA (2017) FDA approves axicabtagene ciloleucel for large B-cell lymphoma. U.S. Food & Drug Administration.
- FDA (2018) FDA approves tisagenlecleucel for adults with relapsed or refractory large B-cell lymphoma. U.S. Food & Drug Administration.
- Bishop D, Xu N, Shen S, O'Brien T (2017) Differing co-stimulatory, linker and spacer domains produce variations in CD4 and CD8 cell composition and cytotoxic potential in CD19-specific chimeric antigen receptor (CAR19) T cells generated with the piggyBac transposase. Cytotherapy 19: S10.
- Bishop DC, Xu N, Tse B, O'Brien TA, Gottlieb DJ (2018) PiggyBac-Engineered T Cells Expressing CD19-Specific CARs that Lack IgG1 Fc spacers have potent activity against B-ALL Xenografts. Mol Ther 26: 1883-1895. [Crossref]
- Dolnikov A, Shen S, Klamer G, Joshi S, Xu N (2015) Antileukemic potency of CD19-specific T cells against chemoresistant pediatric acute lymphoblastic leukemia. Exp Hematol 43: 1001-1014.
- Kebriaei P, Singh H, Huls MH, Figliola MJ, Bassett R (2013) Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. The Journal of clinical investigation 126: 3363-3376.
- ANZCTR (2018) A Phase I Study of CD19 Specific Chimeric Antigen Receptor T-cells for Therapy of Persistent and Relapsed B-cell Leukaemia and Lymphoma Post Allogeneic Stem Cell Transplantation (Registry ID: ACTRN12617001579381). National Health and Medical Research Council Australia.
- ClinicalTrials.gov (2020) Anti-CD19 CAR in PiggyBac Transposon-Engineered T Cells for Relapsed/Refractory B-cell Lymphoma or B-cell Acute Lymphoblastic Leukemia (Registry ID: NCT04289220). Yan'an Affiliated Hospital of Kunming Medical University.
- Grupp SA, Kalos M, Barrett D, Aplenc R (2013) Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368: 1509-1518.
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK (2015) T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385: 517-528.
- Tse B, Xu N, Bishop D, Gottlieb D (2018) Tumour microenvironment modulates cart cell fate in paediatric leukemia. Cytotherapy 20: S97.
- Yan Z, Li L, Wang W, OuYang B, Cheng S (2019) Clinical efficacy and tumor microenvironment influence in a Dose-Escalation study of anti-CD19 Chimeric Antigen Receptor T Cells in Refractory B-Cell Non-Hodgkin's Lymphoma. Clinical Cancer Research 0101.
- Kloss CC, Lee J, Zhang A, Chen F, Melenhorst JJ (2018) Dominant-Negative TGF-beta receptor enhances PSMA-Targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol Ther 26: 1855-1866.
- Slovin SF, Wang X, Hullings M, Arauz G, Bartido S (2013) Chimeric antigen receptor (CAR+) modified T cells targeting prostate-specific membrane antigen (PSMA) in patients (pts) with castrate metastatic prostate cancer (CMPC). Journal of Clinical Oncology 31: 72-72.
- Zhang Q, Helfand BT, Carneiro BA, Qin W (2018) Efficacy against human prostate cancer by prostate-specific membrane antigen-specific, transforming growth factor-β; insensitive genetically targeted cd8+ t-cells derived from patients with metastatic castrate-resistant disease. European Urology 73: 648-652.
- Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z (2006) A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clinical Cancer Research 212: 6106-6115.
- Parente-Pereira AC, Whilding LM, Brewig N, van der Stegen SJ (2013) Synergistic chemoimmunotherapy of epithelial ovarian cancer using erbb-retargeted t cells combined with carboplatin. J Immunol 191: 2437-2445. [Crossref]
- Wang W, Kryczek I, Dostal L, Lin H, Tan L (2016) Effector T cells abrogate stroma-mediated chemoresistance in ovarian cancer. Cell 165: 1092-1105.
- Hamanishi J, Mandai M, Iwasaki M (2007) Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 104: 3360-3365.
- Ko SY, Barengo N, Ladanyi A, Lee J-S, Marini F (2012) HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. The Journal of clinical investigation 122: 3603-3617.
- Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N (2014) Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res 74: 104-118.
- Domschke C, Schneeweiss A, Stefanovic S, Wallwiener M, Heil J, Rom J (2016) Cellular immune responses and immune escape mechanisms in breast cancer: Determinants of immunotherapy. Breast Care (Basel) 11; 102-107. [Crossref]
- Stagg J, Divisekera U, McLaughlin N (2010) Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proceedings of the National Academy of Sciences of the United States of America 107: 1547-1552.
- Morales JK, Kmieciak M, Knutson KL, Bear HD, Manjili MH (2010) GM-CSF is one of the main breast tumor-derived soluble factors involved in the differentiation of CD11b-Gr1- bone marrow progenitor cells into myeloid-derived suppressor cells. Breast Cancer Res Treat 123: 39-49.
- Batchu RB, Gruzdyn OV, Mahmud EM, Chukr F, Dachepalli R (2018) Inhibition of Interleukin-10 in the tumor microenvironment can restore mesothelin chimeric antigen receptor T cell activity in pancreatic cancer in vitro. Surgery 163: 627-632.
- Schueneman AJ, Sugar EA, Uram J, Bigelow E (2013) Low total lymphocyte count is associated with poor survival in patients with resected pancreatic adenocarcinoma receiving a GM-CSF secreting pancreatic tumor vaccine. Ann Surg Oncol 3: S725-S730.
- Maliar A, Servais C, Waks T, Chmielewski M, Lavy R (2012) Cells that target pancreatic adenocarcinoma antigens eliminate tumors and metastases in mice. Gastroenterology 143: 1375-1384 e5.
- Prokopchuk O, Liu Y, Henne-Bruns D, Kornmann M (2005) Interleukin-4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. British journal of cancer 92: 921-928.
- Varghese AM (2017) Chimeric antigen receptor (CAR) T and other T cell strategies for pancreas adenocarcinoma. Chin Clin Oncol 6: 66.
- Neagu MR, Reardon DA (2015) An Update on the role of immunotherapy and vaccine strategies for primary brain tumors. Curr Treat Options Oncol 16: 54.
- Chaudhry K, Shen S, Wang L, Bishop D, Micklethwaite K (2018) Imaging the dynamics of receptor-modified T cell effector function against leukaemia and neuroblastoma targets. Cytotherapy 20: S103.
- Dolnikov A, Xu N, Tse B, Shen S, O'Brien T (2018) Bromodomain inhibitor JQ1 modulates immune suppressive pathways in neuroblastoma and enhances CARGD2T cell therapy. Cytotherapy 20: S97.
- Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV (2005) Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 105: 2862-2868.
- Heylmann D, Bauer M, Becker H (2013) Human CD4+CD25+ regulatory T cells are sensitive to low dose cyclophosphamide: implications for the immune response. PLoS One 8: e83384.
- Traverso I, Fenoglio D, Negrini S, Parodi A, Battaglia F (2012) Cyclophosphamide inhibits the generation and function of CD8(+) regulatory T cells. Hum Immunol 73: 207-213.
- Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D (2004) CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 34: 336-344.
- Kurtulus S, Sakuishi K, Ngiow SF, Joller N (2015) TIGIT predominantly regulates the immune response via regulatory T cells. The Journal of clinical investigation 125: 4053-4062.
- Turk MJ, Guevara-Patiño JA, Rizzuto GA, Engelhorn ME, Houghton AN (2004) Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. The Journal of experimental medicine 200: 771-782.
- Quezada SA, Peggs KS, Simpson TR, Shen Y (2008) Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med 205: 2125-2138.
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA (2013) Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 210: 1695-1710.
- Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A (2010) Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol 40: 22-35.
- Burga RA, Thorn M, Point GR, Guha P, Nguyen CT (2015) Liver myeloid-derived suppressor cells expand in response to liver metastases in mice and inhibit the anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol Immunother 64: 817-829.
- Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF (2012) Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-beta1 signaling pathway. J Immunol 189: 444-453.
- Zhao Q, Kuang DM, Wu Y, Xiao X, Li X-F (2012) Activated CD69+ T Cells foster immune privilege by regulating IDO expression in tumor-associated macrophages. The Journal of Immunology 188: 1117. [Crossref]
- Revoltella RP, Menicagli M, Campani D (2012) Granulocyte-macrophage colony-stimulating factor as an autocrine survival-growth factor in human gliomas. Cytokine 57: 347-359.
- Lakins MA, Ghorani E, Munir H (2018) Cancer-associated fibroblasts induce antigen-specific deletion of CD8 (+) T Cells to protect tumour cells. Nature communications 9: 948-948.
- Zhang Q, Yang XJ, Kundu SD, Pins M (2006) Blockade of transforming growth factor-{beta} signaling in tumor-reactive CD8(+) T cells activates the antitumor immune response cycle. Mol Cancer Ther 5: 1733-1743.
- Li Z, Jiang J, Wang Z, Zhang J, Xiao M (2008) Endogenous Interleukin-4 promotes tumor development by increasing tumor cell resistance to apoptosis. Cancer Research 68: 8687.
- Todaro M, Alea MP, Di Stefano AB, Cammareri P (2007) Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell 1: 389-402.
- Mulligan JK, Rosenzweig SA, Young MR (2010) Tumor secretion of VEGF induces endothelial cells to suppress T cell functions through the production of PGE2. J Immunother 33: 126-135.
- Ninomiya S, Narala N, Huye L, Yagyu S, Savoldo B, Dotti G (2015) Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood 125: 3905-3916.
- Works M, Soni N, Hauskins C, Sierra C, Baturevych A (2019) Anti-B-cell maturation antigen chimeric antigen receptor T cell function against multiple myeloma is enhanced in the presence of lenalidomide. Mol Cancer Ther 18: 2246-2257.
- Bastid J, Regairaz A, Bonnefoy N, Dejou C, Giustiniani J (2015) Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer Immunol Res 3: 254-265.
- Blay J, White TD, Hoskin DW (1997) The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res 57: 2602-2605.
- Bonnefoy N, Bastid J, Alberici G, Bensussan A, Eliaou J-F (2015) CD39: A complementary target to immune checkpoints to counteract tumor-mediated immunosuppression. Oncoimmunology 4: e1003015-e1003015.
- Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R (2014) Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res 20: 4262-4273.
- Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S (2010) Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Research 70: 68-77.
- Hart KM, Byrne KT, Molloy MJ, Usherwood EM, Berwin B (2011) IL-10 immunomodulation of myeloid cells regulates a murine model of ovarian cancer. Frontiers in immunology 2: 29-29.
- Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH (2015) Tumor-Expressed IDO recruits and activates MDSCs in a Treg-Dependent Manner. Cell Rep 13: 412-24.
- Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS (2016) Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest 126: 3130-3144.
- Lu Y, Huang M, Deng T, Zhou X, Yu K (2018) Abstract CT133: A phase I trial of PD-1 deficient engineered T cells with CRISPR/Cas9 in patients with advanced non-small cell lung cancer with PD-L1 expression. Cancer Research 78: CT133.
- Henick BS, Herbst RS, Goldberg SB (2014) The PD-1 pathway as a therapeutic target to overcome immune escape mechanisms in cancer. Expert Opin Ther Targets 18: 1407-1420. [Crossref]
- John LB, Devaud C, Duong CP, Yong CS, Beavis PA (2013) Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res 19: 5636-5646.
- Liu X, Ranganathan R, Jiang S (2016) A Chimeric Switch-Receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res 76: 1578-1590.
- Rosewell Shaw A, Porter CE, Watanabe N (2017) Adenovirotherapy delivering cytokine and checkpoint inhibitor augments CAR T cells against metastatic head and neck cancer. Mol Ther 25: 2440-2451. [Crossref]
- Serganova I, Moroz E, Cohen I, Moroz M, Mane M, Zurita J (2016) Enhancement of PSMA-Directed CAR adoptive immunotherapy by PD-1/PD-L1 blockade. Mol Ther Oncolytics 4: 41-54.
- Gargett T, Yu W, Dotti G, Yvon ES (2016) GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol Ther 24: 1135-1149.
- Li S, Siriwon N, Zhang X, Yang S, Jin T (2017) Enhanced cancer immunotherapy by chimeric antigen receptor-modified T cells engineered to secrete checkpoint inhibitors. Clin Cancer Res 23: 6982-6992.
- ClinicalTrials.gov (2019) CD19-specific CAR-T Cells in CLL/SLL and DLBCL (Registry ID: NCT03960840). Novartis Pharmaceuticals.
- Dolnikov A, Xu N, Tse B, O'Brien T (2018) Epigenetic agent modulates tumour microenvironment and potentiates cart cell therapy. Cytotherapy 20: S97.
- Dolnikov A, Yang S, Shen S, Xu N, Chaudhry K (2016) Prolonging CART cell persistence using conditioning with 5-Azacytidine. Cytotherapy 18: S98.
- Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z (2016) Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood 127: 1117-1127.
- Ramakrishnan R, Huang C, Cho HI, Lloyd M, Johnson J (2012) Autophagy induced by conventional chemotherapy mediates tumor cell sensitivity to immunotherapy. Cancer Res 72: 5483-5493.
- Turtle CJ, Hay KA, Hanafi LA, Li D, Cherian S (2017) Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-Specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol 35: 3010-3020.
- Ohtani H, Orskov AD, Helbo AS, Gillberg L, Liu M (2020) Activation of a subset of evolutionarily young transposable elements and innate immunity are linked to clinical responses to 5-azacytidine. cancer research. Canres 2019: 1696.
- Knox T, Sahakian E, Banik D, Hadley M, Palmer E (2019) Selective HDAC6 inhibitors improve anti-PD-1 immune checkpoint blockade therapy by decreasing the anti-inflammatory phenotype of macrophages and down-regulation of immunosuppressive proteins in tumor cells. Sci Rep 9: 6136.
- Banik D, Moufarrij S, Villagra A (2019) Immunoepigenetics combination therapies: An overview of the role of HDACS in cancer immunotherapy. International journal of molecular sciences 20: 2241.
- van den Bijgaart RJE, Kroesen M, Wassink M, Brok IC (2019) Combined sialic acid and histone deacetylase (HDAC) inhibitor treatment up-regulates the neuroblastoma antigen GD2. J Biol Chem 294: 4437-4449.
- Chen N, Morello A, Tano Z, Adusumilli PS (2016) CAR T-cell intrinsic PD-1 checkpoint blockade: A two-in-one approach for solid tumor immunotherapy. Oncoimmunology 6: e1273302.
- Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H (2015) TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J Clin Invest 125: 2046-2058.
- Michaud M, Xie X, Bravo-San Pedro JM, Zitvogel L (2014) An autophagy-dependent anticancer immune response determines the efficacy of melanoma chemotherapy. Oncoimmunology 3: e944047.
- Rosenberg SA, Dudley ME (2009) Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol 21: 233-240.
- Li AM, Hucks GE, Dinofia AM, Seif AE, Teachey DT (2018) Checkpoint inhibitors augment CD19-directed Chimeric Antigen Receptor (CAR) T cell therapy in relapsed B-Cell acute lymphoblastic leukemia. Blood 132: 556-556.
- Heczey A, Louis CU, Savoldo B, Dakhova O, Durett A, Grilley (2017) CAR T Cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol Ther 25: 2214-2224.
- Zhang P, Zhao S, Wu C, Li J, Li Z (20188) Effects of CSF1R-targeted chimeric antigen receptor-modified NK92MI & T cells on tumor-associated macrophages. Immunotherapy 10: 935-949.
- Sachdeva M, Duchateau P, Depil S, Poirot L, Valton J (2019) Granulocyte-macrophage colony-stimulating factor inactivation in CAR T-cells prevents monocyte-dependent release of key cytokine release syndrome mediators. J Biol Chem 294: 5430-5437.
- Sterner RM, Sakemura R, Cox MJ, Yang N, Khadka RH (2019) GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood 133: 697-709.
- Boroughs AC, Larson RC, Choi BD, Bouffard AA, Riley LS (2019) Chimeric antigen receptor costimulation domains modulate human regulatory T cell function. JCI Insight 4.
- Lynn RC, Matsuyama T, Powell DJ (2015) Targeting FRβ+ tumor associated macrophages with car T cells in ovarian cancer. Journal for Immunotherapy of Cancer 3: 32-32.
- Sakemura R, Cox MJ, Hansen MJ, Hefazi M, Manriquez Roman C, Schick KJ (2019) Targeting cancer associated fibroblasts in the bone marrow prevents resistance to chimeric antigen receptor T Cell therapy in multiple myeloma. Blood 134: 865-865.
- Di S, Zhou M, Pan Z, Sun R, Chen M (2019) Combined Adjuvant of Poly I:C Improves antitumor effects of CAR-T Cells. Frontiers in oncology 9: 241-241.
- Cervantes EV, Boucher JC, Lee SB, Spitler K, Reid K (2019) MDSC Suppression of CAR T cells can be reduced by targeted signaling disruption. Blood 134: 4438-4438.

