Acute and chronic stress both has a profound impact on the immune system and subsequent disease development. However, it is important to understand that their effects are opposite. Acute stress leads to the release of pro-inflammatory cytokines which stimulates the immune system whereas chronic stress leads to immune system suppression. Chronic suppression can lead to viral activation, disease progression and cancer development, specifically, hepatocellular carcinoma. We present a case in which a patient with hepatitis B virus and cirrhosis developed a LI-RADS 4 (probably hepatocellular carcinoma) lesion during a period of substantial stress. Fortunately, his stress was able to be relieved, and he was subsequently found to have regression of this probably malignant lesion to a benign lesion. It is germane for physicians to understand the large impact stress can have on disease development and progression in an effort to curtail this by providing patients with appropriate psychological and emotional support.
hepatitis b virus, cirrhosis, hepatocellular carcinoma, LI-RADS, immune system, stress, regression
The immune system is a complicated and highly regulated network that has been found to be intricately intertwined with hosts’ stress levels. Studies have shown a link between chronic stress and lower natural killer cell and lymphocyte activity, putting patients at risk for viral reactivation. Similarly, associations between diminished stress resilience and cancer development have been established. Herein, we describe the case of a patient with chronic hepatitis B with cirrhosis, who, despite undetectable virus levels and normal serologic markers of liver function, developed a LI-RADS 4 (Liver Imaging Reporting and Data System)* lesion during a period of stress with subsequent regression of the lesion once his stress was relieved. This case demonstrates the impact of the immune system and the hosts’ stress level on disease progression and regression.
A 45-year-old man presented in 1997 to his primary care clinic with a symptom of fatigue. He was found to have an ALT of 150 IU/L and laboratory testing revealed infection with hepatitis B virus (HBV). Recovering from his initial symptoms, he resorted to a traditional herbal medicine for a short time. In 2002, after developing recurrent fatigue, he went to see a gastroenterologist and was diagnosed with liver cirrhosis on an abdominal computed tomography scan (CT). At that time, lamivudine (LAM) was started.
He was first seen in our institution one month after starting LAM. Abdominal magnetic resonance imaging (MRI) demonstrated a nodular liver and small esophageal varices without splenomegaly or ascites. His HBV DNA was positive (no quantitative values were available at that time), HBeAg was negative, anti-HBe was positive and his hepatitis C antibody was negative. Laboratory studies were significant for a normal albumin of 4.4, total bilirubin of 1.7 and platelets of 117,000. After starting LAM 150 milligrams (mg) daily, his HBV DNA became negative (<200 IU/mL), and this medication was continued with subsequent improvement in his ALT and thrombocytopenia.
On June 30, 2008, he was enrolled in a study comparing entecavir and other nucleoside and nucleotide analogues, and was randomized to take adefovir (ADV) 10 mg daily. He remained HBV DNA negative, with normal liver enzymes throughout the study which completed November 1, 2016.
From a psychosocial standpoint, at the initiation of the study, he was feeling generally well, as his virus was well controlled, and he was financially stable as a small business owner. A surveillance MRI on January 13, 2012 demonstrated cirrhosis with medial segment atrophy and caudate hypertrophy and a repeat MRI on October 10, 2014 was stable.
It was at this point in 2014 that his financial stress began, owing to his business closing, and subsequent loss of insurance. He applied for a new job in August 2016 just before the study ended, however, at its completion, was without insurance. This now required him to pay an expensive out of pocket price for his medications, visits to his hepatologist, laboratory studies and abdominal imaging, which added a financially significant burden. Despite these economic constraints, following the completion of study with adefovir, he was switched back to LAM 150 mg daily and took it faithfully.
In early December 2016, he was summoned for physical examination for the job he had applied to, with no guarantee of his hiring. On December 7, 2016, he underwent an MRI as routine follow up. This now showed a new 1.7-centimeter (cm) LI-RADS 4 lesion in segment 8 with hyper enhancement on arterial phase with washout and restricted diffusion (Figure 1). This was the first lesion ever noticed since his primary visit to our clinic in December 2002. In addition to the burden of a likely hepatic malignancy, through the month of December, he was anxiously awaiting a response on the job offering.
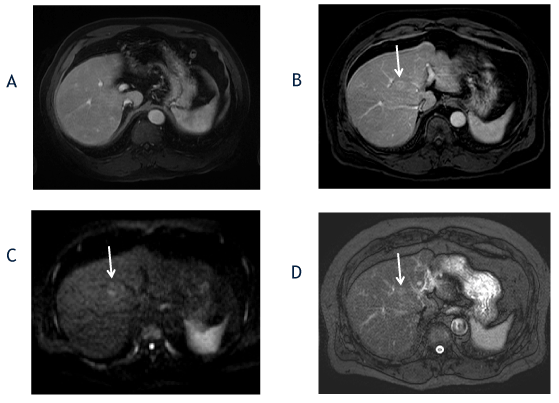
Figure 1. The axial T1-weighted, fat-suppressed postcontrast image from 2014 (A) shows no focal liver lesion. The followup axial T1-weighted, fat-suppressed late arterial postcontrast image from 2016 (B) shows interval development of a hyperenhancing lesion in segment 8 (arrow), which measured 1.7cm and exhibited washout appearance (not shown). The diffusion-weighted (C) and T2-weighted fat-suppressed (D) images show diffusion restriction and mild T2-hyperintensity (ancillary features favoring malignancy).
Finally, in January 2017, he had been offered the position, and, now with insurance coverage, he was switched from LAM to tenofovir (TDF) in April 2017. On April 24, 2017, a repeat MRI to evaluate the lesion demonstrated the same LI-RADS 4 lesion seen previously that was stable in size (Figure 2). These findings were duplicated on ultrasound.
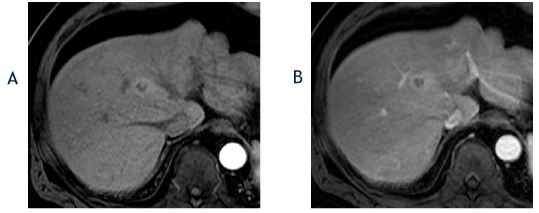
Figure 2. The axial T1-weighted, fat-suppressed arterial phase postcontrast image from April 2017 (A) shows no change in the size of the segment 8 lesion with a hyperenhancing, nodule-in-a-nodule appearance, which is an ancillary feature favoring hepatocellular carcinoma. The corresponding portal phase image (B) shows washout and the lesion was again classified as a LI-RADS 4 observation.
Once accepted to his desired job, despite long hours and busy days, he had increased energy and felt a new sense of security and happiness now that the stress of unemployment was lifted. This mood persisted through subsequent follow up in our clinic. Of note, he never had an elevated ALT, detectable HBV DNA or an elevated AFP that coincided with development of this lesion, or throughout the entire observation period.
Several months later, on October 11, 2017, a follow up MRI showed that the previous LI-RADS 4 observation, now appeared to be LI-RADS 3, with a decrease in size to 1.3-cm, and a change in the lesion’s signal characteristic and enhancement pattern (Figure 3). Subsequent imaging on May 2, 2018 demonstrated a stable in size lesion with increased T2 hyperintensity, and gradual peripheral enhancement, again consistent with a LI-RADS 3 observation (Figure 4).
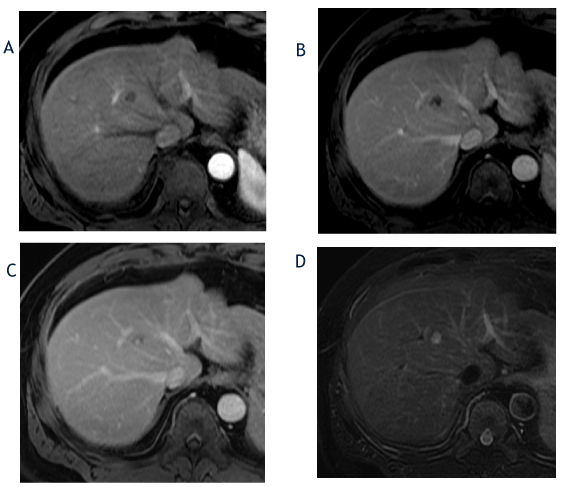
Figure 3. The axial T1-weighted, fat-suppressed arterial phase (A), portal phase (B) and delayed phase (C) images from October 2017 show a decrease in size of the hepatic segment 8 lesion to 1.3cm and a change in the enhancement pattern with lack of arterial-phase hyperenhancement and clumped, peripheral progressive enhancement. The corresponding T2-weighted, fat-suppressed image (D) shows an increase in T2 hyperintensity in the lesion compared with prior imaging. Based on lesion size and lack of hyperenhacement, the lesion was reclassified as a LI-RADS 3 observation.
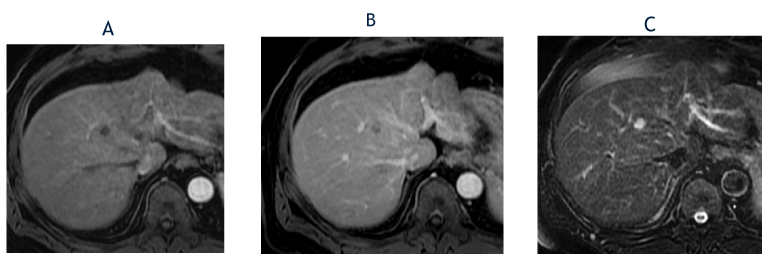
Figure 4. The axial, T1-weighted, fat-suppressed arterial phase postcontrast image from May 2018 (A) shows lack of hyperenhancement in the segment 8 lesion, which is stable in size compared with October 2017. The corresponding delayed phase image (B) shows gradual, clumped peripheral enhancement. The T2-weighted, fat-suppressed image (C) shows further increase in T2 hyperintensity. The lesion was again classified as a LI-RADS 3 observation.
By September 10, 2018, the lesion had lack of enhancement with marked T2 hyperintensity and was reclassified as a LI-RADS 2 observation (Figure 5). Most recently, on January 23, 2019, a repeat MRI showed the lesion had again decreased in size, with similar T2 hyperintensity and gradual progressive enhancement, and was reclassified as LI-RADS 1 observation (Figure 6).
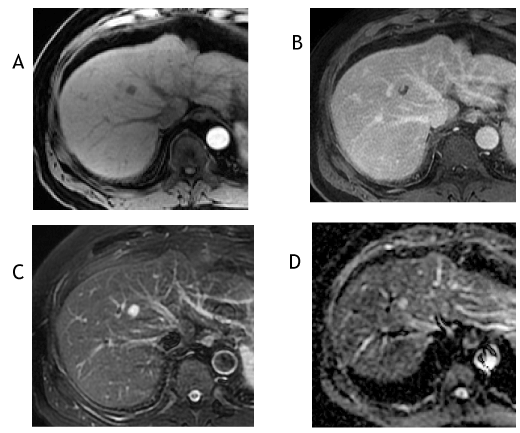
Figure 5. The axial T1-weighted, fat-suppressed arterial phase postcontrast image from September 2018 (A) shows lack of hyperenhancement and the corresponding delayed phase image (B) shows a peripheral clump of enhancement. The T2-weighted, fat-suppressed image (C) shows marked T2 hyperintensity (ancillary feature favoring benignity) and the apparent diffusion coefficient image (D) reveals increased signal, which signifies facilitated diffusion—typically a benign feature. The lesion was reclassified as a LI-RADS 2 observation.
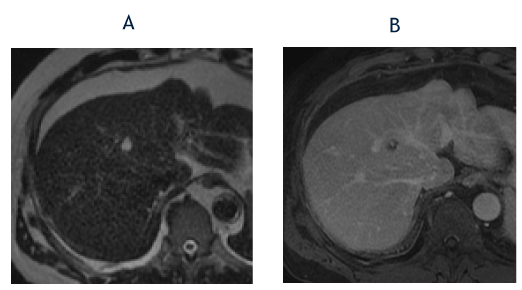
Figure 6. The axial T2-weighted image from January 2019 (A) shows marked hyperintensity in the segment 8 lesion (less strikingly because of the increased T2-weighting compared with Images 4C and 5C). The corresponding T1-weighted, fat-suppressed delayed phase image (B) shows clumped peripheral enhancement similar to recent examinations. The imaging findings are consistent with a slow-filling hemangioma and the lesion was reclassified as a LI-RADS 1 observation.
*The Liver Imaging Reporting and Data System (LI-RADS) system is “a comprehensive system for standardizing the terminology, technique, interpretation, reporting and data collection of liver imaging” most recently updated in 2018 [1]. The LI-RADS system features a 5-point scoring system for “observations” noted on cross-sectional imaging studies in patients with cirrhosis, chronic hepatitis B or current or prior HCC, ranging from LI-RADS 1, or “definitely benign,” to LI-RADS 5, or “definitely HCC” (Figure 7).
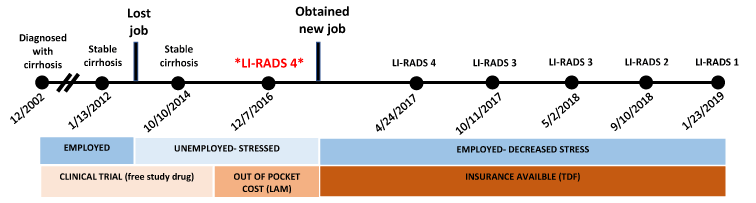
Figure 7. Timeline for patient's clinical course.
- LI-RADS 1 = definitely benign,
- LI-RADS 2 = probably benign,
- LI-RADS 3 = intermediate probability of malignancy,
- LI-RADS 4 = probably HCC and
- LI-RADS 5 = definitely HCC.
The immune response is a complex system that involves cells, proteins, organs and tissues that work together to protect against disease and damage. Acute and chronic stress both impact the immune system, however with opposite downstream effects. Acute stress leads to increased activation of the immune system through release of proinflammatory cytokines [2]. An example of this is the “fight or flight” response that occurs when the body is threatened and leads to the release of adrenaline and glucocorticoids. In contrast, chronic stress can have a detrimental effect on the body, mainly through its suppression of the immune system [3]. This suppression of the immune system can increase the risk for development of disease processes such as cardiovascular, autoimmune, infectious and even neoplastic [4].
Several studies have investigated populations under chronic stress and the exact mechanisms through which the immune system is affected. Specific populations studied include individuals in conflict with significant others, individuals who have suffered from unexpected bereavement and individuals who are long-term caretakers for a spouse with dementia. These studies showed that individuals living with chronic stress had significant immune dysregulation which manifested as slower wound healing, lower natural killer cell and lymphocyte activity and decreased response to vaccines as compared to matched controls [3].
Another consequence of chronic stress and subsequent immune system dysregulation that has been observed is reactivation of latent viruses. This phenomenon is also seen in normal aging and referred to as immunosenescence. Both the innate and adaptive immune systems become less efficient which can lead to an increase in bacterial and viral infections [5,6]. An example of this is exemplified in a study that measured the Epstein Barr Virus (EBV) antibody levels in young adults exposed to sexual abuse. They found that the individuals who had been sexually abused had higher levels of antibodies against EBV signifying a higher degree of viral reactivation [7].
The dynamic between stress and the immune system also has an effect on neoplastic disease development. A study done in Sweden looked at stress resilience and its relationship to cancer development. They conducted a cohort study that looked at men undergoing compulsory military enlistment examinations and measured their psychological stress resilience. They found that men with lower stress resilience had increased risks of liver and lung cancers as compared to those with higher stress resilience. These results were significant even after adjusting for markers of socioeconomic circumstances in childhood [8].
Our case details a patient who suffered from chronic stress related to unemployment and loss of medical insurance. Having chronic hepatitis B with established cirrhosis, our patient was always aware of his risk to develop HCC. In addition to the stress regarding disease progression, the financial burden of indefinite medications, clinic visits, lab work and imaging studies without medical insurance was likely very overwhelming. Interestingly, the timeline of his liver lesion progression and later regression of the probable hepatocellular carcinoma correlated very closely with his financial and emotional stress. Additionally, throughout the years he was followed at our institution, his HBV DNA remained undetectable and his ALT and AFP levels were normal, indicating his virus was well controlled. Therefore, we hypothesize that the differing levels of stress in our patient with chronic liver disease and a constant threat for development of liver cancer contributed to his interesting disease course, and diminished stress levels, in combination with sustained control of viral replication, may have contributed to this regression. In our experience, all patients who present with a LI-RADS 4 lesion with known cirrhosis, always progress to LI-RADS 5, making this case of regression particularly unique. We believe it is imperative that physicians understand the importance that chronic stress can have on patients and their diseases to help enforce stress-reducing practices and implement solutions to prevent disease progression.
Hann HW contributed the conception and design of the manuscript, Chalikonda DM and Shinn BJ wrote the paper and Roth C provided radiographic figures.
Hann HW receives Research grant from Gilead Sciences, Assembly Biosciences and TriHealth. Chalikonda D, Shinn BJ and Roth C have no conflict of interest.
- Elsayes KM, Kielar AZ, Elmohr MM, Chernyak V, Masch WR, et al. (2018) White paper of the Society of Abdominal Radiology hepatocellular carcinoma diagnosis disease-focused panel on LI-RADS v2018 for CT and MRI. Abd Radiol 43: 2625-2642. [Crossref]
- Morey JN, Boggero IA, Scott AB, Segerstrom SC (2015) Current Directions in Stress and Human Immune Function. Curr Opinion Psychol 2015; 5: 13-17. [Crossref]
- Vitlic A, Lord JM, Phillips AC (2014) Stress, ageing and their influence on function, cellular and molecular aspects of the immune system. Age (Dordr) 36: 1169-1185. [Crossref]
- Graham JE, Christian LM, Kiecolt-Glaser JK (2006) Stress, age, and immune function: toward a lifespan approach. J Behav Med 29: 389-400. [Crossref]
- Gomez CR, Boehmer ED, Kovacs EJ (2005) The aging innate immune system. Curr Opin Immunol 17: 457-462. [Crossref]
- Lord JM, Butcher S, Killampali V, Lascelles D, Salmon M (2001) Neutrophil ageing and immunesenescence. Mech Ageing Dev 122: 1521-1535. [Crossref]
- Slopen N, McLaughlin KA, Dunn EC, Koenen KC (2013) Childhood adversity and cell-mediated immunity in young adulthood: does type and timing matter? Brain Behav Immun 28: 63-71. [Crossref]
- Kennedy B, Fang F, Valdimarsdottir U, Udumyan R, Montgomery S, et al. (2017) Stress resilience and cancer risk: a nationwide cohort study. J Epidemiol Community Health 71: 947-953. [Crossref]







